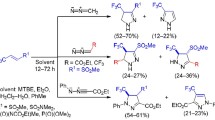
The reaction of N-acylaminophenylcyclopropanes with HNO2 proceeds regioselectively with introduction of an N = O fragment into the three-membered ring and formation of the corresponding Δ2-isoxazolines. For ortho-substituted N-acylaminophenylcyclopropanes side processes were observed, caused by the intramolecular participation of the N-acyl group in conversions of the carbenium ions formed on opening the cyclopropane ring under the action of the nitrosating reagent, and by direct insertion of the modified ortho substituent into the three-membered ring.










Similar content being viewed by others
References
S. S. Mochalov, R. A. Gazzaeva, A. N. Fedotov, Yu. S. Shabarov, and N. S. Zefirov, Khim. Geterotsikl. Soedin., 922 (2003). [Chem. Heterocycl. Comp. 39, 794 (2003)].
A. N. Fedotov, E. V. Trofimova, V. A. Romanov, S. S. Mochalov, Yu. S. Shabarov, and N. S. Zefirov, Khim. Geterotsikl. Soedin., 115 (2008). [Chem. Heterocycl. Comp., 44, 96 (2008)].
E. V. Trofimova, B. P. Archegov, A. N. Fedotov, R. A. Gazzaeva, S. S. Mochalov, and N. S. Zefirov, Khim. Geterotsikl. Soedin., 1368 (2009). [Chem. Heterocycl. Comp., 45, 1095 (2009)].
Yu. S. Shabarov, L. G. Saginova, and R. A. Gazzaeva, Khim. Geterotsikl. Soedin., 738 (1983). [Chem. Heterocycl. Comp., 19, 589 (1983)].
R. A. Gazzaeva, Yu. S. Shabarov, and L. G. Saginova, Khim. Geterotsikl. Soedin., 309 (1984). [Chem. Heterocycl. Comp., 20, 246 (1984)].
L. G. Saginova, I. L. Kukhareva, A. T. Lebedev, and Yu. S. Shabarov, Zh. Org. Khim., 27, 1852 (1991).
O. B. Bondarenko, A. Yu. Gavrilova, L. G. Saginova, N. V. Zyk, and N. S. Zefirov, Izv. Akad. Nauk, Ser. Khim., 3, 741 (2003).
O. B. Bondarenko, A. Yu. Gavrilova, M. A. Kazantseva, V. N. Tikhanushkina, E. E. Nifant’ev, L. G. Saginova, and N. V. Zyk, Zh. Org. Khim., 42, 265 (2006).
A. Z. Kadzhaeva, E. V. Trofimova, A. N. Fedotov, K. A. Potekhin, R. A. Gazzaeva, S. S. Mochalov, and N. S. Zefirov, Khim. Geterotsikl. Soedin., 753 (2009). [Chem. Heterocycl. Comp., 45, 595 (2009)].
A. P. Kozikowski and P. D. Stein, J. Am. Chem. Soc., 104, 4023 (1982).
D. P. Curran, J. Am. Chem. Soc., 105, 5825 (1983).
S. H. Andersen, K. K. Sharma, and K. B. G. Torssell, Tetrahedron, 39, 2241 (1983).
A. P. Kozikowski, Acc. Chem. Res., 17, 410 (1984).
S. Y. Lee, B. S. Lee, C.-W. Lee, and D. Y. Oh, J. Org. Chem., 65, 256 (2000).
A. Yashiro, Y. Nishida, K. Kobayashi, and M. Ohno, Synlett, 361 (2000).
P. Conti, C. Dallanoce, M. De Amici, C. De Micheli, and K. N. Klotz, Bioorg. Med. Chem., 6, 401 (1998).
C. Mohan, G. S. Saharia, and H. R. Sharma, J. Indian Chem. Soc., 53, 181 (1976).
T. Ogawa, M. Inazu, K. Goton, and S. Hayashi, Agents Actions, 31, 321 (1990).
O. A. Reutov, A. L. Kurts, and K. P. Butin, Organicheskaya Khimiya, Vol. 3 [in Russian], BINOM, Moscow (2004), p. 245.
S. Uemara, A. Toshimitsu, M. Okano, J. Chem. Soc., Perkin Trans. 1, 1076 (1978).
S. S. Mochalov, R. A. Gazzaeva, A. N. Fedotov, E. V. Trofimova, I. V. Trushkov, and N. S. Zefirov, Zh. Org. Khim., 40, 1148 (2004).
R. Chang and K. Kim., Tetrahedron Lett., 40, 6773 (1999).
R. Chang and K. Kim., Tetrahedron Lett., 41, 8499 (2000).
Yu. S. Shabarov, V. K. Potapov, and R. Ya. Levina, Zh. Obshch. Khim., 34, 3127 (1964).
R. Ya. Levina, Yu. S. Shabarov, and V. K. Potapov, Zh. Obshch. Khim., 29, 3233 (1959).
S. S. Mochalov, A. N. Fedotov, A. I. Sizov, and Yu. S. Shabarov, Zh. Org. Khim., 15, 1425 (1979).
Yu. S. Shabarov, S. S. Mochalov, I. P. Stepanova, and G. V. Aleksakhin, Dokl. Akad. Nauk, 207, 621 (1972).
Yu. S. Shabarov and S. S. Mochalov, Zh. Org. Khim., 8, 293 (1972).
A. N. Fedotov, I. N. Shishkina, T. G. Kutateladze, S. S. Mochalov, and Yu. S. Shabarov, Khim. Geterotsikl. Soedin., 1063 (1987). [Chem. Heterocycl. Comp., 23, 849 (1987)].
Yu. S. Shabarov and S. S. Mochalov, Zh. Org. Khim., 8, 2085 (1972).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, pp. 1702-1717, November, 2011.
Rights and permissions
About this article
Cite this article
Mochalov, S.S., Gazzaeva, R.A., Kadzhaeva, A.Z. et al. N-acylaminophenylcyclopropanes in reaction with nitrous acid generated in situ . Chem Heterocycl Comp 47, 1415–1429 (2012). https://doi.org/10.1007/s10593-012-0929-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-012-0929-y