Abstract
Cancer is a complex disease, with various pre-existing health ailments enhancing its pathology. In cancer, the extracellular environment contains various intrinsic physiological factors whose levels are altered with aging and pre-existing conditions. In obesity, the tumor microenvironment and metastases are enriched with factors that are both derived locally, and from other physiological compartments. Similarly, in obesity, the cancer cell environment both at the site of origin and at the secondary site i.e., metastatic niche, contains significantly more phenotypically-altered adipocytes than that of un-obese cancer patients. Indeed, obesity has been linked with cancer progression, metastasis, and therapy resistance. Adipocytes not only interact with tumor cells, but also with adjacent stromal cells at primary and metastatic sites. This review emphasizes the importance of bidirectional interactions between adipocytes and breast tumor cells in breast cancer progression and its bone metastases. This paper not only chronicles the role of various adipocyte-derived factors in tumor growth, but also describes the significance of adipocyte-derived bone metastatic factors in the development of bone metastasis of breast cancer. It provides a molecular view of the interplay between the adipocytes and tumor cells involved in breast cancer bone metastasis. However, more research is needed to determine if targeting cancer-associated adipocytes holds promise as a potential therapeutic approach for breast cancer bone metastasis treatment.
Graphic abstract

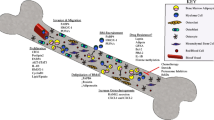
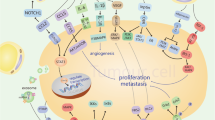
Interplay between adipocytes and breast cancer cells at primary cancer site and metastatic bone microenvironment. AMSC Adipose-derived mesenchymal stem cell, CAA Cancer associated adipocytes, CAF Cancer associated fibroblast, BMSC Bone marrow derived mesenchymal stem cell, BMA Bone marrow adipocyte.



Similar content being viewed by others
References
Andò S et al (2019) Obesity, leptin and breast cancer: epidemiological evidence and proposed mechanisms. Cancers 11(1):62
Ferlay J et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Becker S (2015) A historic and scientific review of breast cancer: the next global healthcare challenge. Int J Gynaecol Obstet 131(Suppl 1):S36–S39
Yu T, Di G (2017) Role of tumor microenvironment in triple-negative breast cancer and its prognostic significance. Chinese J Cancer Res 29(3):237–252
Harbeck N et al (2019) Breast cancer. Nat Rev Dis Primers 5(1):66
Hiraga T (2019) Bone metastasis: Interaction between cancer cells and bone microenvironment. J Oral Biosci 61(2):95–98
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2(8):584–593
Graham N, Qian B-Z (2018) Mesenchymal stromal cells: emerging roles in bone metastasis. Int J Mol Sci 19(4):1121
Hiraga T (2018) Hypoxic microenvironment and metastatic bone disease. Int J Mol Sci 19(11):3523
Pérez-Hernández AI et al (2014) Mechanisms linking excess adiposity and carcinogenesis promotion. Front Endocrinol 5:65
Calle EE, Thun MJ (2004) Obesity and cancer. Oncogene 23(38):6365–6378
Pettersson A, Tamimi RM (2012) Breast fat and breast cancer. Breast Cancer Res Treat 135(1):321–323
Chan DS et al (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 25(10):1901–1914
Emaus A et al (2010) Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res Treat 121(3):651–660
Greenberg AS, Obin MS (2006) Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 83(2):461S-465S
Soysal SD, Tzankov A, Muenst SE (2015) Role of the tumor microenvironment in breast cancer. Pathobiology 82(3–4):142–152
Wang YY et al (2012) Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Lett 324(2):142–151
Park J et al (2014) Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 10(8):455–465
Wang YX et al (2019) Friend or foe: multiple roles of adipose tissue in cancer formation and progression. J Cell Physiol 234(12):21436–21449
Luo L, Liu M (2016) Adipose tissue in control of metabolism. J Endocrinol 231(3):R77–R99
Duong MN et al (2017) The fat and the bad: mature adipocytes, key actors in tumor progression and resistance. Oncotarget 8(34):57622–57641
Balaban S et al (2017) Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab 5:1
Atoum MF, Alzoughool F, Al-Hourani H (2020) Linkage between obesity leptin and breast cancer. Breast Cancer 14:1178223419898458
Lorincz AM, Sukumar S (2006) Molecular links between obesity and breast cancer. Endocr Relat Cancer 13(2):279–292
Munsell MF et al (2014) Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev 36(1):114–136
Esquivel-Velázquez M et al (2015) The role of cytokines in breast cancer development and progression. J interferon Cytokine Res 35(1):1–16
Cabia B et al (2016) A role for novel adipose tissue-secreted factors in obesity-related carcinogenesis. Obes Rev 17(4):361–376
Grossmann ME et al (2010) Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev 29(4):641–653
Dirat BA et al (2010) Unraveling the obesity and breast cancer links: a role for cancer-associated adipocytes? Endocr Dev 19:45–52
Wu Q et al (2019) Cancer-associated adipocytes: key players in breast cancer progression. J Hematol Oncol 12(1):95
Dirat B et al (2011) Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 71(7):2455–2465
Nickel A et al (2018) Adipocytes induce distinct gene expression profiles in mammary tumor cells and enhance inflammatory signaling in invasive breast cancer cells. Sci Rep 8(1):9482–9482
Wang YY et al (2017) Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2(4):e87489
D’Esposito V et al (2012) Adipocyte-released insulin-like growth factor-1 is regulated by glucose and fatty acids and controls breast cancer cell growth in vitro. Diabetologia 55(10):2811–2822
Wang C et al (2015) Human adipocytes stimulate invasion of breast cancer MCF-7 cells by secreting IGFBP-2. PLoS ONE 10(3):e0119348–e0119348
He JY et al (2018) Adipocyte-derived IL-6 and leptin promote breast cancer metastasis via upregulation of Lysyl hydroxylase-2 expression. Cell Commun Signal 16(1):100
Rowan BG et al (2014) Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS ONE 9(2):e89595
Delort L et al (2013) Reciprocal interactions between breast tumor and its adipose microenvironment based on a 3D adipose equivalent model. PLoS ONE 8(6):e66284
Ritter A et al (2015) Characterization of adipose-derived stem cells from subcutaneous and visceral adipose tissues and their function in breast cancer cells. Oncotarget 6(33):34475–34493
Li K et al (2016) Leptin promotes breast cancer cell migration and invasion via IL-18 expression and secretion. Int J Oncol 48(6):2479–2487
Dos Santos E et al (2008) Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep 20(4):971–977
Esquivel-Velazquez M et al (2015) The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res 35(1):1–16
Iyengar P et al (2005) Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Investig 115(5):1163–1176
Christopoulos PF, Msaouel P, Koutsilieris M (2015) The role of the insulin-like growth factor-1 system in breast cancer. Molecular Cancer 14:43–43
Liu E, Samad F, Mueller BM (2013) Local adipocytes enable estrogen-dependent breast cancer growth: role of leptin and aromatase. Adipocyte 2(3):165–169
Grodin JM, Siiteri PK, MacDonald PC (1973) Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab 36(2):207–214
Simpson ER (2003) Sources of estrogen and their importance. J Steroid Biochem Mol Biol 86(3–5):225–230
Miah S et al (2019) Estrogen receptor signaling regulates the expression of the breast tumor kinase in breast cancer cells. BMC Cancer 19(1):78
Rybinska I et al (2020) Adipocytes in breast cancer, the thick and the thin. Cells 9(3):560
Cleland WH, Mendelson CR, Simpson ER (1983) Aromatase activity of membrane fractions of human adipose tissue stromal cells and adipocytes. Endocrinology 113(6):2155–2160
Haynes BP et al (2010) Intratumoral estrogen disposition in breast cancer. Clin Cancer Res 16(6):1790–1801
Díaz-Cruz ES, Shapiro CL, Brueggemeier RW (2005) Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab 90(5):2563–2570
Morris PG et al (2011) Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res 4(7):1021–1029
Kennecke H et al (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277
Amanatullah DF et al (2017) Local estrogen axis in the human bone microenvironment regulates estrogen receptor-positive breast cancer cells. Breast Cancer Res 19(1):121
Frost AR et al (2012) The influence of the cancer microenvironment on the process of metastasis. Int J Breast Cancer 2012:756257
Guyenet SJ, Schwartz MW (2012) Clinical review: regulation of food intake, energy balance, and body fat mass: implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab 97(3):745–755
Hu X et al (2002) Leptin–a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst 94(22):1704–1711
Sánchez-Jiménez F et al (2019) Obesity and breast cancer: role of leptin. Front Oncol 9:596–596
Ishikawa M, Kitayama J, Nagawa H (2004) Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res 10(13):4325–4331
Wu MH et al (2009) Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer 100(4):578–582
Pan H et al (2018) Association between serum leptin levels and breast cancer risk: an updated systematic review and meta-analysis. Medicine 97(27):e11345–e11345
Choi J, Cha YJ, Koo JS (2018) Adipocyte biology in breast cancer: from silent bystander to active facilitator. Prog Lipid Res 69:11–20
Yin N et al (2004) Molecular mechanisms involved in the growth stimulation of breast cancer cells by leptin. Cancer Res 64(16):5870–5875
Zhou W, Guo S, Gonzalez-Perez RR (2011) Leptin pro-angiogenic signature in breast cancer is linked to IL-1 signalling. Br J Cancer 104(1):128–137
Gonzalez-Perez RR et al (2010) Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFkappaB/HIF-1alpha activation. Cell Signal 22(9):1350–1362
Bowers LW et al (2018) Leptin signaling mediates obesity-associated CSC enrichment and EMT in preclinical TNBC models. Mol Cancer Res 16(5):869–879
Templeton ZS et al (2015) Breast cancer cell colonization of the human bone marrow adipose tissue niche. Neoplasia 17(12):849–861
Himbert C et al (2017) Signals from the adipose microenvironment and the obesity-cancer link-a systematic review. Cancer Prev Res 10(9):494–506
Stern JH, Rutkowski JM, Scherer PE (2016) Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab 23(5):770–784
Güven HE et al (2018) Adiponectin: a predictor for breast cancer survival? Eur J Breast Health 15(1):13–17
Dieudonne MN et al (2006) Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun 345(1):271–279
Mauro L et al (2014) Evidences that estrogen receptor alpha interferes with adiponectin effects on breast cancer cell growth. Cell Cycle 13(4):553–564
Masjedi A et al (2018) The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer. Biomed Pharmacother 108:1415–1424
Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89(6):2548–2556
Kim HS et al (2018) IL-6-mediated cross-talk between human preadipocytes and ductal carcinoma in situ in breast cancer progression. J Exp Clin Cancer Res 37(1):200
Gyamfi J et al (2018) Interleukin-6/STAT3 signalling regulates adipocyte induced epithelial-mesenchymal transition in breast cancer cells. Sci Rep 8(1):8859
Liu S et al (2018) HER2 overexpression triggers an IL1α proinflammatory circuit to drive tumorigenesis and promote chemotherapy resistance. Cancer Res 78(8):2040–2051
Cruceriu D et al (2020) The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol 43(1):1–18
Gui Y et al (2017) The association between obesity related adipokines and risk of breast cancer: a meta-analysis. Oncotarget 8(43):75389–75399
Rubio MF et al (2006) TNF-alpha enhances estrogen-induced cell proliferation of estrogen-dependent breast tumor cells through a complex containing nuclear factor-kappa B. Oncogene 25(9):1367–1377
Rivas MA et al (2008) TNF alpha acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent pathways. Exp Cell Res 314(3):509–529
Kim S et al (2008) Berberine suppresses TNF-alpha-induced MMP-9 and cell invasion through inhibition of AP-1 activity in MDA-MB-231 human breast cancer cells. Molecules 13(12):2975–2985
Kang J-H, Yu B-Y, Youn D-S (2007) Relationship of serum adiponectin and resistin levels with breast cancer risk. J Korean Med Sci 22(1):117–121
Acquarone E et al (2019) Resistin: a reappraisal. Mech Ageing Dev 178:46–63
Lee JO et al (2016) Resistin, a fat-derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation. Sci Rep 6:18923
Wang CH et al (2018) Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties. Oncogene 37(5):589–600
Dalamaga M et al (2013) Serum resistin: a biomarker of breast cancer in postmenopausal women? Association with clinicopathological characteristics, tumor markers, inflammatory and metabolic parameters. Clin Biochem 46(7–8):584–590
Ferry G et al (2003) Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem 278(20):18162–18169
D’Souza K et al (2017) Autotaxin is regulated by glucose and insulin in adipocytes. Endocrinology 158(4):791–803
Schmid R et al (2018) ADSCs and adipocytes are the main producers in the autotaxin-lysophosphatidic acid axis of breast cancer and healthy mammary tissue in vitro. BMC Cancer 18(1):1273
Meng G et al (2017) Implications for breast cancer treatment from increased autotaxin production in adipose tissue after radiotherapy. FASEB J 31(9):4064–4077
Shao Y et al (2019) Serum ATX as a novel biomarker for breast cancer. Medicine 98(13):e14973–e14973
Davison Z et al (2011) Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: implications for therapy. Neoplasia 13(6):504–515
Iida M et al (2019) Compensatory role of insulin-like growth factor 1 receptor in estrogen receptor signaling pathway and possible therapeutic target for hormone therapy-resistant breast cancer. Breast Cancer 26(3):272–281
Guaita-Esteruelas S et al (2017) Exogenous FABP4 increases breast cancer cell proliferation and activates the expression of fatty acid transport proteins. Mol Carcinog 56(1):208–217
Hao J et al (2018) Circulating adipose fatty acid binding protein is a new link underlying obesity-associated breast/mammary tumor development. Cell Metab 28(5):689–705
Hancke K et al (2010) Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res Treat 119(2):367–377
Lee YC et al (2011) High visfatin expression in breast cancer tissue is associated with poor survival. Cancer Epidemiol Biomarkers Prev 20(9):1892–1901
Park J, Scherer PE (2012) Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Investig 122(11):4243–4256
Bu D et al (2019) Human endotrophin as a driver of malignant tumor growth. JCI Insight 5(9):e125094
Kaji H (2016) Adipose tissue-derived plasminogen activator inhibitor-1 function and regulation. Compr Physiol 6(4):1873–1896
Carter JC, Church FC (2009) Obesity and breast cancer: the roles of peroxisome proliferator-activated receptor-γ and plasminogen activator inhibitor-1. PPAR Res 2009:345320–345320
Ferroni P et al (2014) Plasma plasminogen activator inhibitor-1 (PAI-1) levels in breast cancer - relationship with clinical outcome. Anticancer Res 34(3):1153–1161
Lopes-Coelho F, Gouveia-Fernandes S, Serpa J (2018) Metabolic cooperation between cancer and non-cancerous stromal cells is pivotal in cancer progression. Tumour Biol 40(2):1010428318756203
Andarawewa KL et al (2005) Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res 65(23):10862–10871
Meng L et al (2001) Tumor necrosis factor alpha and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein alpha and peroxisome proliferator-activated receptor gamma: mechanism of desmoplastic reaction. Cancer Res 61(5):2250–2255
Wang F et al (2014) Mammary fat of breast cancer: gene expression profiling and functional characterization. PLoS ONE 9(10):e109742–e109742
Tan J et al (2011) Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int J Dev Biol 55(7–9):851–859
Bochet L et al (2013) Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res 73(18):5657–5668
Blücher C, Stadler SC (2017) Obesity and breast cancer: current insights on the role of fatty acids and lipid metabolism in promoting breast cancer growth and progression. Front Endocrinol 8:293–293
Martinez-Outschoorn UE et al (2011) Energy transfer in “parasitic” cancer metabolism: mitochondria are the powerhouse and Achilles’ heel of tumor cells. Cell Cycle 10(24):4208–4216
Singh R et al (2016) Increased expression of beige/brown adipose markers from host and breast cancer cells influence xenograft formation in mice. Mol Cancer Res 14(1):78–92
Agustsson T et al (2007) Mechanism of increased lipolysis in cancer cachexia. Cancer Res 67(11):5531–5537
Sun X et al (2020) Fat wasting is damaging: role of adipose tissue in cancer-associated cachexia. Front Cell Dev Biol 8:33–33
Bandyopadhayaya S, Ford B, Mandal CC (2020) Cold-hearted: a case for cold stress in cancer risk. J Therm Biol 91:102608
Argilés JM et al (2014) Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 14(11):754–762
Dalal S (2019) Lipid metabolism in cancer cachexia. Ann Palliat Med 8(1):13–23
Ohno H et al (2012) PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15(3):395–404
Seale P et al (2007) Transcriptional control of brown fat determination by PRDM16. Cell Metab 6(1):38–54
Karamanlidis G et al (2007) C/EBPbeta reprograms white 3T3-L1 preadipocytes to a brown adipocyte pattern of gene expression. J Biol Chem 282(34):24660–24669
Jones LP et al (2011) Abnormal mammary adipose tissue environment of brca1 mutant mice show a persistent deposition of highly vascularized multilocular adipocytes. J Cancer Sci Ther. https://doi.org/10.4172/1948-5956.s2-004
Sanchez-Alvarez R et al (2013) Mitochondrial dysfunction in breast cancer cells prevents tumor growth: understanding chemoprevention with metformin. Cell Cycle 12(1):172–182
Kozlow W, Guise TA (2005) Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia 10(2):169–180
Weilbaecher KN, Guise TA, McCauley LK (2011) Cancer to bone: a fatal attraction. Nat Rev Cancer 11(6):411–425
Blouin S, Basle MF, Chappard D (2008) Interactions between microenvironment and cancer cells in two animal models of bone metastasis. Br J Cancer 98(4):809–815
Wu MY et al (2018) Molecular regulation of bone metastasis pathogenesis. Cell Physiol Biochem 46(4):1423–1438
Sen S et al (2018) Paratharmone related protein (peptide): a novel prognostic, diagnostic and therapeutic marker in head and neck cancer. J Stomatol Oral Maxillofac Surg 119(1):33–36
Zheng L et al (2013) PTHrP expression in human MDA-MB-231 breast cancer cells is critical for tumor growth and survival and osteoblast inhibition. Int J Biol Sci 9(8):830
Chiechi A et al (2013) Role of TGF-β in breast cancer bone metastases. Adv Biosci Biotechnol 4(10C):15–30
Xu C et al (2015) Co-expression of parathyroid hormone related protein and TGF-beta in breast cancer predicts poor survival outcome. BMC Cancer 15:925
Kakonen SM et al (2002) Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem 277(27):24571–24578
Sethi N et al (2011) Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19(2):192–205
Wu X et al (2020) RANKL/RANK system-based mechanism for breast cancer bone metastasis and related therapeutic strategies. Front Cell Dev Biol 8:76–76
Langley RR, Fidler IJ (2011) The seed and soil hypothesis revisited—The role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 128(11):2527–2535
Kang Y et al (2005) Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci 102(39):13909–13914
Morris EV, Edwards CM (2016) The role of bone marrow adipocytes in bone metastasis. J Bone Oncol 5(3):121–123
Diedrich JD et al (2018) The lipid side of bone marrow adipocytes: how tumor cells adapt and survive in bone. Curr Osteoporos Rep 16(4):443–457
Takeshita S et al (2014) Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem 289(24):16699–16710
Luo G, He Y, Yu X (2018) Bone marrow adipocyte: an intimate partner with tumor cells in bone metastasis. Front Endocrinol 9:339
Holt V, Caplan AI, Haynesworth SE (2014) Identification of a subpopulation of marrow MSC-derived medullary adipocytes that express osteoclast-regulating molecules: marrow adipocytes express osteoclast mediators. PLoS ONE 9(10):e108920
Lanotte M, Metcalf D, Dexter TM (1982) Production of monocyte/macrophage colony-stimulating factor by preadipocyte cell lines derived from murine marrow stroma. J Cell Physiol 112(1):123–127
Fan Y et al (2017) Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab 25(3):661–672
Cha YJ, Koo JS (2019) Roles of omental and bone marrow adipocytes in tumor biology. Adipocyte 8(1):304–317
Evangelista GCM et al (2019) 4T1 mammary carcinoma colonization of metastatic niches is accelerated by obesity. Front Oncol 9:685
Herroon MK et al (2013) Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget 4(11):2108–2123
Muruganandan S, Ionescu AM, Sinal CJ (2020) At the crossroads of the adipocyte and osteoclast differentiation programs: future therapeutic perspectives. Int J Mol Sci 21(7):2277
Tencerova M, Kassem M (2016) The bone marrow-derived stromal cells: commitment and regulation of adipogenesis. Front Endocrinol 7:127–127
Li S-N, Wu J-F (2020) TGF-β/SMAD signaling regulation of mesenchymal stem cells in adipocyte commitment. Stem Cell Res Ther 11(1):41
Elsafadi M et al (2019) Convergence of TGFβ and BMP signaling in regulating human bone marrow stromal cell differentiation. Sci Rep 9(1):4977
Ahdjoudj S et al (2005) Transforming growth factor-beta inhibits CCAAT/enhancer-binding protein expression and PPARgamma activity in unloaded bone marrow stromal cells. Exp Cell Res 303(1):138–147
Fajol A, Komaba H (2019) Additional evidence for the role of parathyroid hormone in adipose tissue browning. EBioMedicine 40:3–4
Chan GK et al (2001) PTHrP inhibits adipocyte differentiation by down-regulating PPAR gamma activity via a MAPK-dependent pathway. Endocrinology 142(11):4900–4909
Roca-Rodríguez MM et al (2015) Parathyroid hormone-related protein, human adipose-derived stem cells adipogenic capacity and healthy obesity. J Clin Endocrinol Metab 100(6):E826–E835
Mukherjee S, Aseer KR, Yun JW (2020) Roles of macrophage colony stimulating factor in white and brown adipocytes. Biotechnol Bioprocess Eng 25(1):29–38
Hofbauer LC, Schoppet M (2004) Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 292(4):490–495
An JJ et al (2007) Expression and regulation of osteoprotegerin in adipose tissue. Yonsei Med J 48(5):765–772
Zhang L et al (2016) Role of osteoprotegerin (OPG) in bone marrow adipogenesis. Cell Physiol Biochem 40(3–4):681–692
Kühn MC et al (2012) Adipocyte-secreted factors increase osteoblast proliferation and the OPG/RANKL ratio to influence osteoclast formation. Mol Cell Endocrinol 349(2):180–188
Zaky DS et al (2019) Circulating osteoprotegerin level in relation to obesity in middle aged females. Int J Prev Treat 8(2):41–45
Ashley DT et al (2011) Similar to adiponectin, serum levels of osteoprotegerin are associated with obesity in healthy subjects. Metabolism 60(7):994–1000
Treeck O, Buechler C, Ortmann O (2019) Chemerin and cancer. Int J Mol Sci 20(15):3750
Muruganandan S et al (2013) Chemerin neutralization blocks hematopoietic stem cell osteoclastogenesis. Stem Cells 31(10):2172–2182
Thommesen L et al (2006) Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J Cell Biochem 99(3):824–834
Timaner M, Tsai KK, Shaked Y (2020) The multifaceted role of mesenchymal stem cells in cancer. Semin Cancer Biol 60:225–237
Berebichez-Fridman R, Montero-Olvera PR (2018) Sources and clinical applications of mesenchymal stem cells: state-of-the-art review. Sultan Qaboos Univ Med J 18(3):e264–e277
Ghosh S et al (2014) Association of obesity and circulating adipose stromal cells among breast cancer survivors. Mol Biol Rep 41(5):2907–2916
Hillers LE et al (2018) Obesity-activated adipose-derived stromal cells promote breast cancer growth and invasion. Neoplasia 20(11):1161–1174
Strong AL et al (2013) Obesity associated alterations in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways. Breast Cancer Res 15(5):R102
Benova A, Tencerova M (2020) Obesity-induced changes in bone marrow homeostasis. Front Endocrinol 11:294–294
Halade GV et al (2011) Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp Gerontol 46(1):43–52
Goldstein RH et al (2010) Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Can Res 70(24):10044–10050
Blache U et al (2019) Mesenchymal stromal cell activation by breast cancer secretomes in bioengineered 3D microenvironments. Life Sci Alliance 2(3):e201900304
Mishra PJ et al (2008) Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res 68(11):4331–4339
Hill BS et al (2017) Tumor-educated mesenchymal stem cells promote pro-metastatic phenotype. Oncotarget 8(42):73296–73311
Martin FT et al (2010) Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat 124(2):317–326
Dwyer RM et al (2007) Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res 13(17):5020–5027
Karnoub AE et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449(7162):557–563
Shangguan L et al (2012) Inhibition of TGF-β/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells 30(12):2810–2819
Orimo A et al (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121(3):335–348
Strong AL et al (2017) Obesity enhances the conversion of adipose-derived stromal/stem cells into carcinoma-associated fibroblast leading to cancer cell proliferation and progression to an invasive phenotype. Stem Cells Int 2017:9216502
Cao JJ (2011) Effects of obesity on bone metabolism. J Orthop Surg Res 6:30
Ayoub NM et al (2019) Impact of obesity on clinicopathologic characteristics and disease prognosis in pre-and postmenopausal breast cancer patients: a retrospective institutional study. J Obesity. https://doi.org/10.1155/2019/3820759
Osman MA, Hennessy BT (2015) Obesity correlation with metastases development and response to first-line metastatic chemotherapy in breast cancer. Clin Med Insights Oncol 9:105–112
Yazici O et al (2014) Effect of body mass index on metastatic pattern in early breast cancer patients. J Clin Oncol 32(15_suppl):e11553
Ewertz M et al (2011) Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol 29(1):25–31
White AJ et al (2015) Overall and central adiposity and breast cancer risk in the sister study. Cancer 121(20):3700–3708
Neuhouser ML et al (2015) Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol 1(5):611–621
Kamineni A et al (2013) Body mass index, tumor characteristics, and prognosis following diagnosis of early-stage breast cancer in a mammographically screened population. Cancer Causes Control 24(2):305–312
Daling JR et al (2001) Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer 92(4):720–729
Majed B et al (2008) Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat 111(2):329–342
Santa-Maria CA et al (2015) Aggressive estrogen-receptor-positive breast cancer arising in patients with elevated body mass index. Int J Clin Oncol 20(2):317–323
Suzuki R et al (2006) Body weight and postmenopausal breast cancer risk defined by estrogen and progesterone receptor status among Swedish women: a prospective cohort study. Int J Cancer 119(7):1683–1689
Tehard B, Clavel-Chapelon F (2005) Several anthropometric measurements and breast cancer risk: results of the E3N cohort study. Int J Obesity 30(1):156–163
Canchola AJ et al (2012) Body size and the risk of postmenopausal breast cancer subtypes in the California teachers study cohort. Cancer Causes Control. https://doi.org/10.1007/s10552-012-9897-x
Vona-Davis L et al (2008) Triple-negative breast cancer and obesity in a rural appalachian population. Cancer Epidemiol Biomarkers Prev 17(12):3319–3324
Yuan HJ, Sun KW, Yu K (2014) Leptin promotes the proliferation and migration of human breast cancer through the extracellular-signal regulated kinase pathway. Mol Med Rep 9(1):350–354
Walter M et al (2009) Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene 28(30):2745–2755
Gyamfi J et al (2018) Multifaceted roles of interleukin-6 in adipocyte-breast cancer cell interaction. Transl Oncol 11(2):275–285
Cai X et al (2017) Inflammatory factor TNF-α promotes the growth of breast cancer via the positive feedback loop of TNFR1/NF-κB (and/or p38)/p-STAT3/HBXIP/TNFR1. Oncotarget 8(35):58338–58352
Martin TJ (2005) Osteoblast-derived PTHrP is a physiological regulator of bone formation. J Clin Investig 115(9):2322–2324
Soki FN, Park SI, McCauley LK (2012) The multifaceted actions of PTHrP in skeletal metastasis. Future Oncol 8(7):803–817
Le Pape F, Vargas G, Clézardin P (2016) The role of osteoclasts in breast cancer bone metastasis. J Bone Oncol 5(3):93–95
Juarez P, Guise TA (2011) TGF-beta in cancer and bone: implications for treatment of bone metastases. Bone 48(1):23–29
Yagiz K, Rittling SR (2009) Both cell-surface and secreted CSF-1 expressed by tumor cells metastatic to bone can contribute to osteoclast activation. Exp Cell Res 315(14):2442–2452
Christopoulos PF, Msaouel P, Koutsilieris M (2015) The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer 14:43
Rieunier G et al (2019) Bad to the bone: the role of the insulin-like growth factor axis in osseous metastasis. Clin Cancer Res 25(12):3479–3485
Fritton JC et al (2010) The insulin-like growth factor-1 binding protein acid-labile subunit alters mesenchymal stromal cell fate. J Biol Chem 285(7):4709–4714
Mulholland BS, Forwood MR, Morrison NA (2019) Monocyte chemoattractant protein-1 (MCP-1/CCL2) drives activation of bone remodelling and skeletal metastasis. Curr Osteoporos Rep 17(6):538–547
Ito A et al (2008) Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J Biol Chem 283(51):35715–35723
Acknowledgements
Dr. Chandi C. Mandal is supported by the Department of Biotechnology [6242-P9/RGCB/PMD/DBT/CCML/2015], University Grant Commissions [30-49/2014 (BSR)] and Department of Science and Technology (India)-Russian Foundation for Basic Research (INT/RUS/RFBR/P-256). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. We make an apology to various authors whose works could not be cited because of several limitations and space constraints.
Author information
Authors and Affiliations
Contributions
SS had prepared tables, figures, and drafted manuscript. MT had re-organised and edited manuscript critically. CCM had formulated the manuscript and edited and contributed to the manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
All authors have declared no conflict of interest to this manuscript or closely related manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soni, S., Torvund, M. & Mandal, C.C. Molecular insights into the interplay between adiposity, breast cancer and bone metastasis. Clin Exp Metastasis 38, 119–138 (2021). https://doi.org/10.1007/s10585-021-10076-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-021-10076-0