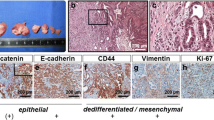
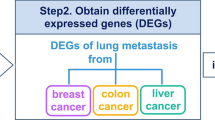
Abstract
We have previously shown that metastases are generally characterized by a core program of gene expression that induces the oxidative energy metabolism, activates vascularization/tissue remodeling, silences extracellular matrix interactions, and alters ion homeostasis. This core program distinguishes metastases from their originating primary tumors as well as from their target host tissues. We hypothesized that organ preference is reflected in additional, site-selective components within the metastatic gene expression programs. Expanding our prior analysis of 653 human gene expression profiles plus data from a murine model, we find that the release from the primary tumor is associated with a suppression of functions that are important for the identity of the organ of origin, such as a down-regulation of steroid hormone responsiveness in the disseminated foci derived from prostate cancer. Metastases adjust to their target microenvironment by up-regulating—even overexpressing—genes and genetic programs that are characteristic of that organ. Finally, alterations in RNA and protein processing as well as immune deviation are common. In the clinic, metastases are mostly treated with the chemotherapy protocols devised for their primary tumors. Adjustments that account for the gene expression differences between primary and metastatic cancers have the potential to improve the currently dismal success rates.



Similar content being viewed by others
References
Weber GF (2007) Molecular mechanisms of cancer. Springer, Dordrecht
Fidler IJ (1975) Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res 35:218–224
Weber GF, Ashkar S (2000) Stress response genes—the genes that make cancer metastasize. J Mol Med 78:404–408
Zhang G, He B, Weber GF (2003) Growth factor signaling induces metastasis genes in transformed cells. A molecular connection between Akt kinase and osteopontin in breast cancer. Mol Cell Biol 23:6507–6519
Whalen KA, Weber GF, Benjamin TL, Schaffhausen BS (2008) Polyomavirus middle T antigen induces the transcription of osteopontin, a gene important for migration of transformed cells. J Virol 82:4946–4954
Mesa C Jr, Mirza M, Mitsutake N, Sartor M, Medvedovic M, Tomlinson C, Knauf JA, Weber GF, Fagin JA (2006) Conditional activation of the RET/PTC3 and B-RAFV600E oncoproteins in thyroid cells is associated with unique patterns of gene expression that predict distinct functional properties and a preferential role of BRAF in extracellular matrix remodeling. Cancer Res 66:6521–6529
Weber GF (2008) Molecular mechanisms of metastasis. Cancer Lett 270:181–190
Turpeenniemi-Hujanen T, Thorgeirsson UP, Hart IR, Grant SS, Liotta LA (1985) Expression of collagenase IV (basement membrane collagenase) activity in murine tumor cell hybrids that differ in metastatic potential. J Natl Cancer Inst 75:99–103
Matrisian LM, Bowden GT, Krieg P, Furstenberger G, Briand JP, Leroy P, Breathnach R (1986) The mRNA coding for the secreted protease transin is expressed more abundantly in malignant than in benign tumors. Proc Natl Acad Sci USA 83:9413–9417
Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Hausmann I, Matzku S, Wenzel A, Ponta H, Herrlich P (1991) A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65:13–24
Senger DR, Perruzzi CA, Papadopoulos A (1989) Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation. Anticancer Res 9:1291–1299
Steeg PS, Bevilacqua G, Kopper L (1988) Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 80:200–204
Alvarez OA, Carmichael DF, DeClerck YA (1990) Inhibition of collagenolytic activity and metastasis of tumor cells by a recombinant human tissue inhibitor of metalloproteinases. J Natl Cancer Inst 82:589–595
Hartung F, Wang Y, Aronow B, Weber GF (2017) A core program of gene expression characterizes cancer metastases. Oncotarget 8:102161–102175
Mueller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410:50–56
Streeter PR, Berg EL, Rouse BT, Bargatze RF, Butcher EC (1988) A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature 331:41–46
Ruoslahti E (2000) Targeting tumor vasculature with homing peptides from phage display. Semin Cancer Biol 10:435–442
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18
Lê Cao K-A, Rohart F, McHugh L, Korn O, Wells CA (2014) YuGene: a simple approach to scale gene expression data derived from different platforms for integrated analyses. Genomics 103:239–251
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412
Huang S, Eleniste PP, Wayakanon K, Mandela P, Eipper BA, Mains RE, Allen MR, Bruzzaniti A (2014) The Rho-GEF Kalirin regulates bone mass and the function of osteoblasts and osteoclasts. Bone 60:235–245
Church RH, Ali I, Tate M, Lavin D, Krishnakumar A, Kok HM, Hombrebueno JR, Dunne PD, Bingham V, Goldschmeding R, Martin F, Brazil DP (2017) Gremlin1 plays a key role in kidney development and renal fibrosis. Am J Physiol Renal Physiol 312:F1141–F1157
Zhifang M, Liang W, Wei Z, Bin H, Rui T, Nan W, Shuhai Z (2015) The androgen receptor plays a suppressive role in epithelial- mesenchymal transition of human prostate cancer stem progenitor cells. BMC Biochem 16:13
Zhu ML, Kyprianou N (2010) Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. FASEB J 24:769–777
de Launoit Y, Baert JL, Chotteau-Lelievre A, Monte D, Coutte L, Mauen S, Firlej V, Degerny C, Verreman K (2006) The Ets transcription factors of the PEA3 group: transcriptional regulators in metastasis. Biochim Biophys Acta 1766:79–87
Selvaraj A, Prywes R (2004) Expression profiling of serum inducible genes identifies a subset of SRF target genes that are MKL dependent. BMC Mol Biol 5:13
Agarwal P, Verzi MP, Nguyen T, Hu J, Ehlers ML, McCulley DJ, Xu SM, Dodou E, Anderson JP, Wei ML, Black BL (2011) The MADS box transcription factor MEF2C regulates melanocyte development and is a direct transcriptional target and partner of SOX10. Development 138:2555–2565
Schummer P, Kuphal S, Vardimon L, Bosserhoff AK, Kappelmann M (2016) Specific c-Jun target genes in malignant melanoma. Cancer Biol Ther 17:486–497
Efimenko E, Padua MB, Manuylov NL, Fox SC, Morse DA, Tevosian SG (2013) The transcription factor GATA4 is required for follicular development and normal ovarian function. Dev Biol 381:144–158
Heikinheimo M, Ermolaeva M, Bielinska M, Rahman NA, Narita N, Huhtaniemi IT, Tapanainen JS, Wilson DB (1997) Expression and hormonal regulation of transcription factors GATA-4 and GATA-6 in the mouse ovary. Endocrinology 138:3505–3514
Vihma H, Pruunsild P, Timmusk T (2008) Alternative splicing and expression of human and mouse NFAT genes. Genomics 92:279–291
Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM (2009) Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr 19:109–124
Tian L, Yu X (2015) Lipid metabolism disorders and bone dysfunction—interrelated and mutually regulated (Review). Mol Med Rep 12:783–794
Li M, Li A, Zhou S, Xu Y, Xiao Y, Bi R, Yang W (2018) Heterogeneity of PD-L1 expression in primary tumors and paired lymph node metastases of triple negative breast cancer. BMC Cancer 18:4
Manson QF, Schrijver WAME, Ter Hoeve ND, Moelans CB, van Diest PJ (2019) Frequent discordance in PD-1 and PD-L1 expression between primary breast tumors and their matched distant metastases. Clin Exp Metastasis 36:29–37
Bissonnette RP, Echeverri F, Mahboubi A, Green DR (1992) Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature 359:552–554
Koskinen PJ, Alitalo K (1993) Role of myc amplification and overexpression in cell growth, differentiation and death. Semin Cancer Biol 4:3–12
Weber GF (2016) Metabolism in cancer metastasis. Int J Cancer 138:2061–2066
Salami J, Crews CM (2017) Waste disposal—an attractive strategy for cancer therapy. Science 355:1163–1167
Kaplan RN, Riba RD, Zacharoulis S (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827
McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, Weinberg RA (2008) Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 133:994–1005
Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, Maru Y (2008) The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nature Cell Biol 10:1349–1355
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D (2015) Tumor exosome integrins determine organotropic metastasis. Nature 527:329–335
Bae JS, Oh AR, Lee HJ, Ahn YH, Cha JY (2016) Hepatic Elovl6 gene expression is regulated by the synergistic action of ChREBP and SREBP-1c. Biochem Biophys Res Commun 478:1060–1066
Liu Z, Ukomadu C (2008) Fibrinogen-like protein 1, a hepatocyte derived protein is an acute phase reactant. Biochem Biophys Res Commun 365:729–734
Sheftel AD, Stehling O, Pierik AJ, Elsässer HP, Mühlenhoff U, Webert H, Hobler A, Hannemann F, Bernhardt R, Lill R (2010) Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc Natl Acad Sci USA 107:11775–11780
Acknowledgements
This research was supported by DOD grant PR094070 to GFW. The breast cancer portion also received support from the Marlene Harris-Ride Cincinnati/Pilot Program to GFW. We are thankful to Conover Talbot, The Johns Hopkins School of Medicine, Institute for Basic Biomedical Sciences, for the principal component analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hartung, F., Patil, A., Meshram, R.J. et al. Gene expression signatures of site-specificity in cancer metastases. Clin Exp Metastasis 37, 159–171 (2020). https://doi.org/10.1007/s10585-019-09995-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-019-09995-w