Abstract
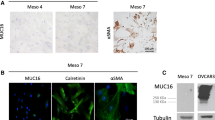
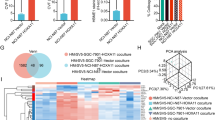
Epithelial ovarian cancer (EOC) is considered to secrete various factors in order to promote peritoneal dissemination through cell-to-cell interaction between cancer and mesothelial cells. We previously revealed that TGF-β secreted from EOC induces normal human peritoneal mesothelial cells (HPMCs) to differentiate into cancer-associated mesothelial cells (CAMCs). However, the relationship between tumor cells and CAMCs in EOC is still unclear. We hypothesized that CAMCs also secrete chemokines that attract cancer cells and induce peritoneal dissemination of EOC. We examined chemokines secreted from HPMCs and CAMCs by human chemokine array, and revealed that conditioned medium of CAMCs (CAMCs-CM) included many types of chemokines. The signals of CCL2 were the highest compared with other chemokines. The secretion and relative expression of CCL2 were significantly higher in CAMCs. Recombinant CCL2 promoted trans-mesothelial migration of HPMCs and the migration and invasion by EOC cells. In addition, CCL2 secreted from CAMCs promoted invasion of EOC cells. Furthermore, the neutralizing antibody of CCL2 reduced invasion by EOC. Clinical outcomes of patients whose tissue expressed higher CCR2 were significantly poorer than in patients whose tissue expression was lower. CCL2 activated the phosphorylation of p38 mitogen-activated protein kinase (MAPK). In addition, CAMCs-CM activated the p38 MAPK pathway. Phosphorylation of p38 MAPK reduced with the presence of neutralizing antibody of CCL2. In conclusion, these data indicate CCL2 in CAMCs-CM promoted the malignant potential of EOC. CCL2 plays a crucial role in the tumor microenvironment of EOC.






Similar content being viewed by others
References
Colombo N, Peiretti M, Parma G et al (2010) Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(Suppl 5):v23–30
Torre LA, Islami F, Siegel RL et al (2017) Global cancer in women: burden and trends. Cancer Epidemiol Biomark Prev 26:444–457
Patient report from Gynecologic Oncology Committee (2016) Acta obstetrica et gynaecologica. Japonica 68:1117–1160
Kikkawa F, Nawa A, Ino K et al (2006) Advances in treatment of epithelial ovarian cancer. Nagoya J Med Sci 68:19–26
Brown PO, Palmer C (2009) The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med 6:e1000114
Chang LC, Huang CF, Lai MS et al (2018) Prognostic factors in epithelial ovarian cancer: a population-based study. PLoS One 13:e0194993
Pannu HK, Bristow RE, Montz FJ, Fishman EK (2003) Multidetector CT of peritoneal carcinomatosis from ovarian cancer. Radiographics 23:687–701
Force USPST, Grossman DC, Curry SJ et al (2018) Screening for ovarian cancer: US preventive services task force recommendation statement. JAMA 319:588–594
Drabsch Y, ten Dijke P (2012) TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metast Rev 31:553–568
Pickup M, Novitskiy S, Moses HL (2013) The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer 13:788–799
Yu Y, Xiao CH, Tan LD et al (2014) Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-beta signalling. Br J Cancer 110:724–732
Giannoni E, Bianchini F, Masieri L et al (2010) Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res 70:6945–6956
Fujikake K, Kajiyama H, Yoshihara M et al (2018) A novel mechanism of neovascularization in peritoneal dissemination via cancer-associated mesothelial cells affected by TGF-beta derived from ovarian cancer. Oncol Rep 39:193–200
Kajiyama H, Shibata K, Ino K et al (2007) Possible involvement of SDF-1alpha/CXCR4-DPPIV axis in TGF-beta1-induced enhancement of migratory potential in human peritoneal mesothelial cells. Cell Tissue Res 330:221–229
Sugiyama K, Kajiyama H, Shibata K et al (2014) Expression of the miR200 family of microRNAs in mesothelial cells suppresses the dissemination of ovarian cancer cells. Mol Cancer Ther 13:2081–2091
Liu J, Chen S, Wang W et al (2016) Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-beta pathways. Cancer Lett 379:49–59
Worzfeld T, Pogge von Strandmann E, Huber M et al (2017) The unique molecular and cellular microenvironment of ovarian cancer. Front Oncol 7:24
Muralidhar GG, Barbolina MV (2013) Chemokine receptors in epithelial ovarian cancer. Int J Mol Sci 15:361–376
Sarvaiya PJ, Guo D, Ulasov I et al (2013) Chemokines in tumor progression metastasis. Oncotarget 4:2171–2185
EssenBioScience-IncuCyte ZOOM live cell imaging.
Dobrzycka B, Mackowiak-Matejczyk B, Terlikowska KM et al (2013) Serum levels of IL-6, IL-8 and CRP as prognostic factors in epithelial ovarian cancer. Eur Cytokine Netw 24:106–113
Popple A, Durrant LG, Spendlove I et al (2012) The chemokine, CXCL12, is an independent predictor of poor survival in ovarian cancer. Br J Cancer 106:1306–1313
O'Connor T, Borsig L, Heikenwalder M (2015) CCL2-CCR2 signaling in disease pathogenesis. Endocr Metab Immune Disord Drug Targets 15:105–118
Li S, Lu J, Chen Y et al (2017) MCP-1-induced ERK/GSK-3beta/Snail signaling facilitates the epithelial-mesenchymal transition and promotes the migration of MCF-7 human breast carcinoma cells. Cell Mol Immunol 14:621–630
Lin TH, Liu HH, Tsai TH et al (2013) CCL2 increases alphavbeta3 integrin expression and subsequently promotes prostate cancer migration. Biochim Biophys Acta 1830:4917–4927
Furukawa S, Soeda S, Kiko Y et al (2013) MCP-1 promotes invasion and adhesion of human ovarian cancer cells. Anticancer Res 33:4785–4790
Tsaur I, Rutz J, Makarevic J et al (2015) CCL2 promotes integrin-mediated adhesion of prostate cancer cells in vitro. World J Urol 33:1051–1056
Eferl R (2013) CCL2 at the crossroad of cancer metastasis. JAKSTAT 2:e23816
Fang WB, Jokar I, Zou A et al (2012) CCL2/CCR2 chemokine signaling coordinates survival and motility of breast cancer cells through Smad3 protein- and p42/44 mitogen-activated protein kinase (MAPK)-dependent mechanisms. J Biol Chem 287:36593–36608
He S, He S, Chen CH et al (2015) The chemokine (CCL2-CCR2) signaling axis mediates perineural invasion. Mol Cancer Res 13:380–390
Chien J, Neums L, Powell A et al (2018) Genetic evidence for early peritoneal spreading in pelvic high-grade serous cancer. Front Oncol 8:58
Pasquier J, Gosset M, Geyl C et al (2018) CCL2/CCL5 secreted by the stroma induce IL-6/PYK2 dependent chemoresistance in ovarian cancer. Mol Cancer 17:47
Solinas G, Germano G, Mantovani A, Allavena P (2009) Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86:1065–1073
Negus RP, Stamp GW, Relf MG et al (1995) The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest 95:2391–2396
Hefler L, Tempfer C, Heinze G et al (1999) Monocyte chemoattractant protein-1 serum levels in ovarian cancer patients. Br J Cancer 81:855–859
Mikula-Pietrasik J, Uruski P, Szubert S et al (2016) Biochemical composition of malignant ascites determines high aggressiveness of undifferentiated ovarian tumors. Med Oncol 33:94
Tsuyada A, Chow A, Wu J et al (2012) CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res 72:2768–2779
O'Hayre M, Salanga CL, Handel TM, Allen SJ (2008) Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J 409:635–649
Pahler JC, Tazzyman S, Erez N et al (2008) Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia 10:329–340
Green JM, Alvero AB, Kohen F, Mor G (2009) 7-(O)-Carboxymethyl daidzein conjugated to N-t-Boc-hexylenediamine: a novel compound capable of inducing cell death in epithelial ovarian cancer stem cells. Cancer Biol Ther 8:1747–1753
Puvanenthiran S, Essapen S, Seddon AM, Modjtahedi H (2016) Impact of the putative cancer stem cell markers and growth factor receptor expression on the sensitivity of ovarian cancer cells to treatment with various forms of small molecule tyrosine kinase inhibitors and cytotoxic drugs. Int J Oncol 49:1825–1838
Sun W, Li WJ, Wei FQ et al (2016) Blockade of MCP-1/CCR4 signaling-induced recruitment of activated regulatory cells evokes an antitumor immune response in head and neck squamous cell carcinoma. Oncotarget 7:37714–37727
Fader AN, Rasool N, Vaziri SA et al (2010) CCL2 expression in primary ovarian carcinoma is correlated with chemotherapy response and survival outcomes. Anticancer Res 30:4791–4798
Saito T, Katabuchi H (2016) Annual Report of the Committee on Gynecologic Oncology, Japan Society of Obstetrics and Gynecology: patient annual report for 2013 and treatment annual report for 2008. J Obstet Gynaecol Res 42: 1069–1079
Wolf MJ, Hoos A, Bauer J et al (2012) Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell 22:91–105
Mellado M, Rodriguez-Frade JM, Aragay A et al (1998) The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J Immunol 161:805–813
Zhuang H, Cao G, Kou C, Liu T (2018) CCL2/CCR2 axis induces hepatocellular carcinoma invasion and epithelial-mesenchymal transition in vitro through activation of the Hedgehog pathway. Oncol Rep 39:21–30
Acknowledgements
We sincerely thank Mr. Y. Koya and Y. Yamakita (Bell Research Center, Department of Obstetrics and Gynecology Collaborative Research, Nagoya University Graduate School of Medicine) for various forms of technical support.
Funding
This study was supported by JSPS (Japan Society for the Promotion of Science) KAKENHI Grants-in-Aid for Scientific Research: Grant Numbers 17H04338 and 16K15704.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
10585_2019_9993_MOESM3_ESM.jpg
Fig. S1 The expressions of CCR2 in EOC cell lines. The protein levels of SKOV-3, A-2780, and OVCAR-3 were high. However, that of ES-2 was low (JPG 76 kb)
10585_2019_9993_MOESM4_ESM.jpg
Fig. S2 Proliferation assay to assess the effect of CCL2 for the cell proliferation. There was no correlation between CCL2 and the cell proliferation (JPG 224 kb)
10585_2019_9993_MOESM5_ESM.jpg
Fig. S3 Survival analyses on stratification by each histological type of EOC. A: overall survival (serous carcinoma), B: overall survival (clear-cell carcinoma), C: progression-free survival (serous carcinoma), D: progression-free survival (clear-cell carcinoma) (JPG 430 kb)
10585_2019_9993_MOESM6_ESM.jpg
Fig. S4 Schematic summary of the current study. Enhanced production of CCL2 from CAMCs is crucial for the development of peritoneal carcinomatosis of EOC through the adhesion-, migration-, and invasion-promoting effects of tumor cells. These findings indicate the scheme of the auto-stimulatory triangle axis among cancer, ascites, and the mesothelium (JPG 528 kb)
Rights and permissions
About this article
Cite this article
Yasui, H., Kajiyama, H., Tamauchi, S. et al. CCL2 secreted from cancer-associated mesothelial cells promotes peritoneal metastasis of ovarian cancer cells through the P38-MAPK pathway. Clin Exp Metastasis 37, 145–158 (2020). https://doi.org/10.1007/s10585-019-09993-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-019-09993-y