Abstract
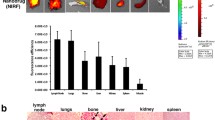
Metastatic disease accounts for most deaths due to breast cancer and thus identification of novel ways to prevent this complication remains a key goal. A frequently employed preclinical model of breast cancer metastasis relies on xenografted human MDA-MB-231 cells, since these reliably produce both soft tissue and osseous metastases when introduced into the arterial circulation of athymic mice. Herein, we explored the ability of suramin (SA), an agent shown to antagonize the effects of various stromal cell-derived growth factors relevant to bone marrow colonization of tumor cells, administered both with and without paclitaxel (PTX), to inhibit the development of MDA-MB-231 metastases. Treatment with SA, PTX, or PTX plus SA (PTX/SA) was begun either at day-1, or 7 days after intra-arterial inoculation of luciferase-expressing MDA-MB-231-luc2 cells. Using in vivo and ex vivo bioluminescence imaging to detect macro-metastases, we found that PTX/SA treatment initiated on day-1 was able to dramatically reduce the frequency of bone metastases. PTX/SA and PTX administration commenced at day 7, in contrast, had no significant effect on the frequency of bone metastases, but exerted a relatively modest inhibitory effect on growth of metastases. Interestingly, reminiscent of what is seen clinically in anti-HER2 treated individuals, several of the PTX/SA-treated long term survivors went on to develop late onset CNS metastasis. Our results suggest that combining SA with PTX either in an adjuvant setting or during medical interventions that can increase the numbers of circulating tumour cells might be an effective way to prevent the development of metastases.





Similar content being viewed by others
Abbreviations
- BLI:
-
Bioluminescence imaging
- CNS:
-
Central nervous system
- FBS:
-
Fetal bovine serum
- H&E:
-
Hematoxylin and eosin staining
- MST:
-
Median survival time
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NIH-III:
-
NIH-III (nu/nu;beige/beige) mice
- PBS:
-
Phosphate-buffered saline
- PTX:
-
Paclitaxel
- ROI:
-
Region of interest
- SA:
-
Suramin
References
Nguyen DX, Bos PD, Massagué J (2009) Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9(4):274–284
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Rabbani SA, Mazar AP (2007) Evaluating distant metastases in breast cancer: from biology to outcomes. Cancer Metastasis Rev 26:663–674
Akhtari M, Mansuri J, Newman KA, Guise TM, Seth P (2008) Biology of breast cancer bone metastasis. Cancer Biol Ther 7:3–9
Bussard KM, Gay CV, Mastro AM (2008) The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev 27:41–55
Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massagué J (2009) Genes that mediate breast cancer metastasis to the brain. Nature 459:1005–1009
Lu J, Steeg PS, Price JE, Krishnamurthy S, Mani SA, Reuben J, Cristofanilli M, Dontu G, Bidaut L, Valero V, Hortobagyi GN, Yu D (2009) Breast cancer metastasis: challenges and opportunities. Cancer Res 69:4951–4953
Damia G, D’Incalci M (2009) Contemporary pre-clinical development of anticancer agents-what are the optimal preclinical models? Eur J Cancer 45:2768–2781
Abu-Khalaf MM, Harris LN (2011) Anti-microtubule agents. In: DeVita VT, Rosenberg SA, Lawrence TS (eds) Cancer: principles and practice of Oncology, 9th edn. Lippincott Williams and Wilkins, Philadelphia, pp 413–420
Burstein HJ, Harris JR, Morrow M (2011) Malignant tumors of the breast. In: DeVita VT, Lawrence TS, Rosenberg SA (eds) In cancer: principles and practice of Oncology, 9th edn. Lippincott Williams and Wilkins, Philadelphia, pp 1401–1439
McGeary RP, Bennett AJ, Tran QB, Cosgrove KL, Ross BP (2008) Suramin: clinical uses and structure-activity relationships. Mini Rev Med Chem. 8:1384–1394
Garrett JS, Coughlin SR, Niman HL, Tremble PM, Giels GM, Williams LT (1984) Blockade of autocrine stimulation in simian sarcoma virus-transformed cells reverses down-regulation of platelet-derived growth factor receptors. Proc Natl Acad Sci U S A 81:7466–7470
Pollak M, Richard M (1990) Suramin blockade of insulin like growth factor I-stimulated proliferation of human osteosarcoma cells. J Natl Cancer Inst 82:1349–1352
Kathir KM, Kumar TK, Yu C (2006) Understanding the mechanism of the anti-mitogenic activity of suramin. Biochemistry 45:899–906
Hosang M (1985) Suramin binds to platelet-derived growth factor and inhibits its biological activity. J Cell Biochem 29:265–273
Song S, Wientjes MG, Walsh C, Au JL (2001) Nontoxic doses of suramin enhance activity of paclitaxel against lung metastases. Cancer Res 61:6145–6150
Lu Z, Wientjes TS, Au JL (2005) Nontoxic suramin treatments enhance docetaxel activity in chemotherapy-pretreated non-small cell lung xenograft tumors. Pharm Res 22:1069–1078
Song S, Wientjes MG, Gan Y, Au JL (2000) Fibroblast growth factors: an epigenetic mechanism of broad spectrum resistance to anticancer drugs. Proc Natl Acad Sci U S A 97:8658–8663
Pesenti E, Sola F, Mongelli N, Grandi M, Spreafico F (1992) Suramin prevents neovascularisation and tumour growth through blocking of basic fibroblast growth factor activity. Br J Cancer 66:367–372
Meads MB, Gatenby RA, Dalton WS (2009) Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer 9:665–674
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Bondareva A, Downey CM, Ayres F, Liu W, Boyd SK, Hallgrimsson B, Jirik FR (2009) The lysyl oxidase inhibitor, beta-aminopropionitrile, diminishes the metastatic colonization potential of circulating breast cancer cells. PLoS ONE 4:e5620
Song S, Yu B, Wei Y, Wientjes MG, Au JL (2004) Low-dose suramin enhanced paclitaxel activity in chemotherapy-naive and paclitaxel-pretreated human breast xenograft tumors. Clin Cancer Res 10:6058–6065
Tran TA, Gillet L, Roger S, Besson P, White E, Le Guennec JY (2009) Non-anti-mitotic concentrations of taxol reduce breast cancer cell invasiveness. Biochem Biophys Res Commun 379:304–308
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147:275–292
Castells M, Thibault B, Delord JP, Couderc B (2012) Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. Int J Mol Sci 13:9545–9571
Meads MB, Hazlehurst LA, Dalton WS (2008) The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res 14:2519–2526
Lustberg MB, Pant S, Ruppert AS, Shen T, Wei Y, Chen L, Brenner L, Shiels D, Jensen RR, Berger M, Mrozek E, Ramaswamy B, Grever M, Au JL, Wientjes MG, Shapiro CL (2012) Phase I/II trial of non-cytotoxic suramin in combination with weekly paclitaxel in metastatic breast cancer treated with prior taxanes. Cancer Chemother Pharmacol 70(1):49–56
Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhänel M, Spruss T, Bernhardt G, Graeff C, Färber L, Gschaidmeier H, Buschauer A, Fricker G (2002) Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest 110:1309–1318
Kemper EM, van Zandbergen AE, Cleypool C, Mos HA, Boogerd W, Beijnen JH, van Tellingen O (2003) Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin Cancer Res 9:2849–2855
Burstein HJ, Lieberman G, Slamon DJ, Winer EP, Klein P (2005) Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Ann Oncol 16:1772–1777
Tinhofer I, Hristozova T, Stromberger C, Keilhoiz U, Budach V (2012) Monitoring of circulating tumor cells and their expression of EGFR/phospho-EGFR during combined radiotherapy regimens in locally advanced squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 83:e685–e690
Choy A, McCulloch P (1996) Induction of tumour cell shedding into effluent venous blood breast cancer surgery. Br J Cancer 73:79–82
Galán M, Viñolas N, Colomer D, Soler G, Muñoz M, Longarón R, Ventura PJ, Gascón P, Estapé J (2002) Detection of occult breast cancer cells by amplification of CK19 mRNA by reverse transcriptase-polymerase chain reaction: role of surgical manipulation. Anticancer Res 22:2877–2884
Acknowledgments
This study was supported by a Breast Cancer Translational Group Grant from the Alberta Cancer Foundation. F.R.J. was the recipient of a Canada Research Chairs award.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Arvind K.Singla and Alla Bondareva have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Singla, A.K., Bondareva, A. & Jirik, F.R. Combined treatment with paclitaxel and suramin prevents the development of metastasis by inhibiting metastatic colonization of circulating tumor cells. Clin Exp Metastasis 31, 705–714 (2014). https://doi.org/10.1007/s10585-014-9661-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-014-9661-6