Abstract
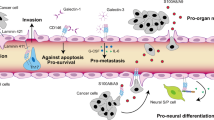
Expression of the L1 cell adhesion molecule (L1CAM) is frequently increased in cancer patients compared to healthy individuals and also linked with bad prognosis of solid tumours. Previously, we could show that full-length L1CAM promotes metastasis formation via up-regulation of gelatinolytic activity in fibrosarcoma. In this study, we aimed to extend this finding to haematogenous malignancies and carcinomas, and to specifically elucidate the impact of L1CAM on major steps of the metastatic cascade. In a well-established T-cell lymphoma spontaneous metastasis model, silencing of L1CAM significantly improved survival of the mice, while intradermal tumour growth remained unaltered. This correlated with significantly decreased spontaneous metastasis formation. L1CAM suppression abrogated the metastatic potential of T-cell lymphoma as well as carcinoma cells as demonstrated by reduced migration and invasion in vitro and reduced formation of experimental metastasis in vivo. At the molecular level, silencing of L1CAM led to reduced expression of gelatinases MMP-2 and -9 in vitro and decreased gelatinolytic activity in primary tumours and metastases in vivo. In accordance, knock down of L1CAM had similar suppressive effects on migration, invasion and in vivo-gelatinolytic activity as treatment with the specific gelatinase inhibitor SB-3CT. This newly discovered impact of L1CAM on distinct steps of the metastatic cascade and MMP activity highlights the potential of possible L1CAM-directed therapies to inhibit metastatic spread.






Similar content being viewed by others
Abbreviations
- CAM:
-
Cell adhesion molecule
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DMSO:
-
Dimethyl sulfoxide
- IgSF:
-
Immunoglobulin superfamily
- i.d.:
-
Intradermal
- i.v.:
-
Intravenous
- L1CAM:
-
L1 cell adhesion molecule
- MMP-2:
-
Matrix metalloproteinase-2
- MMP-9:
-
Matrix metalloproteinase-9
- PCNA:
-
Proliferating cell nuclear antigen
- PE:
-
Phycoerythrin
- shRNA:
-
Short hairpin RNA
- X-Gal:
-
5-Bromo-4-chloro-indolyl-β-d-galactopyranoside
References
Sporn MB (1996) The war on cancer. Lancet 347:1377–1381
Fidler IJ (2003) The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer 3:453–458
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147:275–292
Pals ST, de Gorter DJJ, Spaargaren M (2007) Lymphoma dissemination: the other face of lymphocyte homing. Blood 110:3102–3111
Noël A, Gutiérrez-Fernández A, Sounni NE et al (2012) New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Front Pharmacol 3:140
Deryugina EI, Quigley JP (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25:9–34
Mook ORF, Frederiks WM, Van Noorden CJF (2004) The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta 1705:69–89
Zeng ZS, Cohen AM, Guillem JG (1999) Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis 20:749–755
Dufour A, Zucker S, Sampson NS et al (2010) Role of matrix metalloproteinase-9 dimers in cell migration: design of inhibitory peptides. J Biol Chem 285:35944–35956
Dufour A, Sampson NS, Zucker S, Cao J (2008) Role of the hemopexin domain of matrix metalloproteinases in cell migration. J Cell Physiol 217:643–651
Chetty C, Vanamala SK, Gondi CS et al (2012) MMP-9 induces CD44 cleavage and CD44 mediated cell migration in glioblastoma xenograft cells. Cell Signal 24:549–559
Gerg M, Kopitz C, Schaten S et al (2008) Distinct functionality of tumor cell-derived gelatinases during formation of liver metastases. Mol Cancer Res 6:341–351
Brown S, Bernardo M, Li Z-H (2000) Potent and selective mechanism-based inhibition of gelatinases. J Am Chem Soc 122:6799–6800
Krüger A, Arlt MJE, Gerg M et al (2005) Antimetastatic activity of a novel mechanism-based gelatinase inhibitor. Cancer Res 65:3523–3526
Westermarck J, Kähäri VM (1999) Regulation of matrix metalloproteinase expression in tumor invasion. Faseb J 13:781–792
Munshi HG, Stack MS (2006) Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev 25:45–56
Denzel S, Mack B, Eggert C et al (2012) MMP7 is a target of the tumour-associated antigen EpCAM. Int J Exp Pathol 93:341–353
Cavallaro U, Christofori G (2004) Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 4:118–132
Kiefel H, Bondong S, Hazin J et al (2012) L1CAM: a major driver for tumor cell invasion and motility. Cell Adh Migr 6:374–384
Wai Wong C, Dye DE, Coombe DR (2012) The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int J Cell Biol 2012:340296
Gavert N, Ben-Shmuel A, Raveh S, Ben-Ze’ev A (2008) L1-CAM in cancerous tissues. Expert Opin Biol Ther 8:1749–1757
Raveh S, Gavert N, Ben-Ze’ev A (2009) L1 cell adhesion molecule (L1CAM) in invasive tumors. Cancer Lett 282:137–145
Fogel M, Gutwein P, Mechtersheimer S et al (2003) L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet 362:869–875
Schäfer H, Geismann C, Heneweer C et al (2012) Myofibroblast-induced tumorigenicity of pancreatic ductal epithelial cells is L1CAM dependent. Carcinogenesis 33:84–93
Gavert N, Sheffer M, Raveh S et al (2007) Expression of L1-CAM and ADAM10 in human colon cancer cells induces metastasis. Cancer Res 67:7703–7712
Gavert N, Ben-Shmuel A, Lemmon V et al (2010) Nuclear factor-kappaB signaling and ezrin are essential for L1-mediated metastasis of colon cancer cells. J Cell Sci 123:2135–2143
Gavert N, Vivanti A, Hazin J et al (2011) L1-mediated colon cancer cell metastasis does not require changes in EMT and cancer stem cell markers. Mol Cancer Res 9:14–24
Thies A, Schachner M, Moll I et al (2002) Overexpression of the cell adhesion molecule L1 is associated with metastasis in cutaneous malignant melanoma. Eur J Cancer 38:1708–1716
Arlt MJE, Novak-Hofer I, Gast D et al (2006) Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res 66:936–943
Fischer E, Grünberg J, Cohrs S et al (2012) L1-CAM-targeted antibody therapy and (177)Lu-radioimmunotherapy of disseminated ovarian cancer. Int J Cancer 130:2715–2721
Knogler K, Grünberg J, Zimmermann K et al (2007) Copper-67 radioimmunotherapy and growth inhibition by anti-L1-cell adhesion molecule monoclonal antibodies in a therapy model of ovarian cancer metastasis. Clin Cancer Res 13:603–611
Hai J, Zhu C-Q, Bandarchi B et al (2012) L1 cell adhesion molecule promotes tumorigenicity and metastatic potential in non-small cell lung cancer. Clin Cancer Res 18:1914–1924
Hung S-C, Wu I-H, Hsue S-S et al (2010) Targeting l1 cell adhesion molecule using lentivirus-mediated short hairpin RNA interference reverses aggressiveness of oral squamous cell carcinoma. Mol Pharm 7:2312–2323
Krüger A, Schirrmacher V, von Hoegen P (1994) Scattered micrometastases visualized at the single-cell level: detection and re-isolation of lacZ-labeled metastasized lymphoma cells. Int J Cancer 58:275–284
Soneoka Y, Cannon PM, Ramsdale EE et al (1995) A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res 23:628–633
Yee JK, Miyanohara A, LaPorte P et al (1994) A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA 91:9564–9568
Kopitz C, Anton M, Gansbacher B, Krüger A (2005) Reduction of experimental human fibrosarcoma lung metastasis in mice by adenovirus-mediated cystatin C overexpression in the host. Cancer Res 65:8608–8612
Krüger A, Umansky V, Rocha M et al (1994) Pattern and load of spontaneous liver metastasis dependent on host immune status studied with a lacZ transduced lymphoma. Blood 84:3166–3174
Arlt M, Kopitz C, Pennington C et al (2002) Increase in gelatinase-specificity of matrix metalloproteinase inhibitors correlates with antimetastatic efficacy in a T-cell lymphoma model. Cancer Res 62:5543–5550
Wolterink S, Moldenhauer G, Fogel M et al (2010) Therapeutic antibodies to human L1CAM: functional characterization and application in a mouse model for ovarian carcinoma. Cancer Res 70:2504–2515
Gast D, Riedle S, Riedle S et al (2005) L1 augments cell migration and tumor growth but not beta3 integrin expression in ovarian carcinomas. Int J Cancer 115:658–665
Gast D, Riedle S, Issa Y et al (2008) The cytoplasmic part of L1-CAM controls growth and gene expression in human tumors that is reversed by therapeutic antibodies. Oncogene 27:1281–1289
Zhang H, Wong CCL, Wei H et al (2012) HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene 31:1757–1770
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2:563–572
Luzzi KJ, MacDonald IC, Schmidt EE et al (1998) Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 153:865–873
Sebens Müerköster S, Werbing V, Sipos B et al (2007) Drug-induced expression of the cellular adhesion molecule L1CAM confers anti-apoptotic protection and chemoresistance in pancreatic ductal adenocarcinoma cells. Oncogene 26:2759–2768
Stoeck A, Gast D, Sanderson MP et al (2007) L1-CAM in a membrane-bound or soluble form augments protection from apoptosis in ovarian carcinoma cells. Gynecol Oncol 104:461–469
Bergmann F, Wandschneider F, Sipos B et al (2010) Elevated L1CAM expression in precursor lesions and primary and metastastic tissues of pancreatic ductal adenocarcinoma. Oncol Rep 24:909–915
Friedl P, Alexander S (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147:992–1009
Yilmaz M, Christofori G (2010) Mechanisms of motility in metastasizing cells. Mol Cancer Res 8:629–642
Björklund M, Koivunen E (2005) Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta 1755:37–69
Gavert N, Conacci-Sorrell M, Gast D et al (2005) L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol 168:633–642
Tsutsumi S, Morohashi S, Kudo Y et al (2011) L1 Cell adhesion molecule (L1CAM) expression at the cancer invasive front is a novel prognostic marker of pancreatic ductal adenocarcinoma. J Surg Oncol 103:669–673
Zecchini S, Bianchi M, Colombo N et al (2008) The differential role of L1 in ovarian carcinoma and normal ovarian surface epithelium. Cancer Res 68:1110–1118
Acknowledgments
The authors thank Stephanie Hauser, Laura Bickel and Mareike Lehnhoff (all three from Institute for Experimental Oncology and Therapy Research, Klinikum rechts der Isar, Technische Universität München, Germany) for their expert technical support. This work was supported by the Deutsche Forschungsgemeinschaft (Grants KR2047/1-1 and 1-2) to Achim Krüger.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dirk Weinspach and Bastian Seubert have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10585_2013_9613_MOESM1_ESM.ppt
Supplementary Fig. 1 Tumour cell-derived L1CAM expression is an important regulator of the metastatic potential in vitro. L-CI.5s cells were transduced with retroviruses containing a shRNA sequence directed against murine L1CAM (shL1-3) or with a non-targeting shRNA sequence (shscr, scrambled shRNA). a TaqMan® analysis revealed significantly reduced L1CAM expression in L-CI.5s cells. Mean expression of L1CAM mRNA ± SEM (columns ± bars) in L-CI.5s cells. L1CAM mRNA expression levels were normalised to 18S rRNA levels and the mean of the reference group (shscr) was set as 100 % (n = 3; shL1-3 versus shscr: **p = 0.007, as determined by unpaired t-test). b Western blot analysis of immunoprecipitated L1CAM revealed that L1CAM protein levels were significantly reduced in L-CI.5s cells. c Flow cytometric analysis revealed that L1CAM expression was significantly reduced in L-CI.5s cells. d For analysis of cell viability/proliferation, 2 × 103 cells/well were seeded in 96 well plates, and the number of living cells was quantified 0, 24, 48, 72 and 96 h after seeding using the AlamarBlue® proliferation assay (n = 6). e Mean number of migrated cells per image section ± SEM (columns ± bars) for the different L-CI.5s cells as analysed by trans-well migration assay. The mean of the reference group (shscr) was set as 100 % (n = 6; shL1-3 vs. shscr: ***p ≤ 0.001, as determined by unpaired t-test). f Mean number of invaded cells per image section ± SEM (columns ± bars) for the different L-CI.5s cells as analysed by Matrigel invasion assay. The mean of the reference group (shscr) was set as 100 % (n = 6; shL1-3 vs. shscr: **p = 0.005, unpaired t-test). g Mean MMP-2 mRNA expression ± SEM (columns ± bars) in the different L-CI.5s cells as determined by TaqMan analysis. MMP-2 levels were normalised to 18S rRNA levels and the mean of the reference group (shscr) was set as 100 % (n = 3; shL1-3 vs. shscr: *p = 0.028, as determined by unpaired t-test). h Mean MMP-9 mRNA expression ± SEM (columns ± bars) in the different L-CI.5s cells as determined by TaqMan analysis. MMP-9 levels were normalised to 18S rRNA levels and the mean of the reference group (shscr) was set as 100 %. (n = 3; shL1-3 vs. shscr: *p = 0.015, as determined by unpaired t-test). i Mean number of migrated cells per image section ± SEM (columns ± bars) for the different L-CI.5s cells as determined by trans-well migration assay. Cells were either incubated with 20 µM gelatinase inhibitor SB-3CT or DMSO as a control. The mean of the reference group (shscr, DMSO) was set as 100 % (n = 6 each; all group comparison: **p ≤ 0.001; shscr, SB-3CT vs. shscr, DMSO: *p < 0.05; shL1-3, DMSO vs. shscr, DMSO: p > 0.05; shL1-3, SB-3CT vs. shscr, DMSO: *p < 0.05; shL1-3, SB-3CT vs. shL1-3, DMSO: p > 0.05, as determined by Kruskal–Wallis One Way ANOVA on Ranks). j Mean number of invaded cells per image section ± SEM (columns ± bars) for the different L-CI.5s cells as determined by Matrigel invasion assay. Cells were either incubated with 20 μM gelatinase inhibitor SB-3CT or DMSO as a control. The mean of the reference group (shscr, DMSO) was set as 100 % (n = 6 each; all group comparison: *p ≤ 0.001, as determined by One Way ANOVA; single group comparison: shscr, SB-3CT vs. shscr, DMSO: ***p < 0.001; shL1-3, DMSO vs. shscr, DMSO: ***p < 0.001; shL1-3, SB-3CT vs. shscr, DMSO: ***p < 0.001; shL1-3, SB-3CT vs. shL1-2, DMSO: p = 0.090; as determined by One Way ANOVA and subsequent post hoc comparison by Holm–Sidak method). Supplementary material 1 (PPT 646 kb)
10585_2013_9613_MOESM2_ESM.ppt
Supplementary Fig. 2 a Respective close-up picture showing X-Gal staining of a liver from a mouse that has not been inoculated with tumour cells. b X-Gal staining was performed on cryo-sections of primary tumours (upper panel) and livers bearing metastases (lower panel) originating from the L-CI.5s spontaneous metastasis assay (bars: 100 μm). Supplementary material 2 (PPT 10466 kb)
10585_2013_9613_MOESM3_ESM.ppt
Supplementary Fig. 3 L1CAM positively regulates the metastatic potential of ovarian carcinoma cells. SKOV3ip-lacZ cells were transduced with retroviruses containing a shRNA sequence directed against human L1CAM (shL1-3) or with a non-targeting shRNA sequence (shscr, scrambled shRNA). a TaqMan® analysis revealed significantly reduced L1CAM expression in L-CI.5s cells. Mean expression of L1CAM mRNA ± SEM (columns ± bars) in SKOV3ip-lacZ cells. L1CAM mRNA expression levels were normalised to 18S rRNA levels and the mean of the reference group (shscr) was set as 100 % (n = 3; shL1-3 vs. shscr: ***p ≤ 0.001, as determined by unpaired t-test). b Western blot analysis revealed that L1CAM protein levels were significantly reduced in SKOV3ip-lacZ cells. c Flow cytometric analysis revealed that L1CAM expression was significantly reduced in SKOV3ip-lacZ cells. d Mean number of migrated cells per image section ± SEM (columns ± bars) for the different SKOV3ip-lacZ cells as analysed by trans-well migration assay. Cells were either incubated with 20 µM gelatinase inhibitor SB-3CT or DMSO as a control. The mean of the reference group (shscr, DMSO) was set as 100 % (n = 6 each; all group comparison: ***p ≤ 0.001, as determined by One Way ANOVA; single group comparison: shscr, SB-3CT vs. shscr, DMSO: ***p < 0.001; shL1-3, DMSO vs. shscr, DMSO: ***p ≤ 0.001; shL1-3, SB-3CT vs. shscr, DMSO: ***p < 0.001; shL1-3, SB-3CT vs. shL1-3, DMSO: *p = 0.048, as determined by One Way ANOVA and subsequent post hoc comparison by Holm-Sidak method). e Mean number of invaded cells per image section ± SEM (columns ± bars) for the different SKOV3ip-lacZ cells as determined by Matrigel invasion assay. Cells were either incubated with 20 μM gelatinase inhibitor SB-3CT or DMSO as a control. The mean of the reference group (shscr, DMSO) was set as 100 % (n = 6 each; all group comparison: ***p ≤ 0.001, as determined by Kruskal–Wallis One Way ANOVA; single group comparison: shscr, SB-3CT vs. shscr, DMSO: *p > 0.05; shL1-3, DMSO vs. shscr, DMSO: *p < 0.05; shL1-3, SB-3CT vs. shscr, DMSO: *p < 0.05; shL1-3, SB-3CT vs. shL1-3, DMSO: not testable, as determined by Kruskal–Wallis One Way ANOVA on Ranks and subsequent post hoc comparison by Dunn’s method). f For analysis of cell viability/proliferation, 2 × 103 cells/well were seeded in 96 well plates, and the number of living cells was quantified 0 h, 24 h, 48 h, 72 h and 96 h after seeding using the AlamarBlue® proliferation assay (n = 6). Supplementary material 3 (PPT 802 kb)
Rights and permissions
About this article
Cite this article
Weinspach, D., Seubert, B., Schaten, S. et al. Role of L1 cell adhesion molecule (L1CAM) in the metastatic cascade: promotion of dissemination, colonization, and metastatic growth. Clin Exp Metastasis 31, 87–100 (2014). https://doi.org/10.1007/s10585-013-9613-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-013-9613-6