Abstract
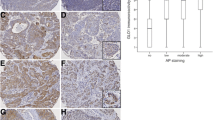
Oral squamous cell carcinoma (OSCC) account for more than 90 % of all oral malignant lesions. Lactate dehydrogenase 5 (LDH5) has the highest efficiency among all other isoenzymes to catalyse pyruvate transformation to lactate and is significantly overexpressed in several different tumour entities. LDH5 overexpression confers an advantage on malignant cells, allows them to grow faster, and to metastasize. No data regarding LDH5 expression and OSCC outcome are available. Expression of LDH5 was analysed in OSCC specimen (n = 191) and cancer cell lines (BICR3, BICR56) by immunohistochemistry, real-time quantitative reverse transcription-PCR (RT-PCR) analysis, and western blotting. Scanned images were digitally analysed using ImageJ and the immunomembrane plug-in. LDH5 expression on protein level was correlated with clinicopathological characteristics and impact on survival. LDH5 was co-labelled with glucose transporter-1 (GLUT-1), Ki-67, and hypoxia inducible factor 1 (HIF-1α) in immunohistochemical double staining experiments. Expression subgroups were identified by receiver operating characteristics analysis. LDH5 expression was significantly associated with tumour progression, and recurrence of the tumour. Multivariate analysis demonstrated LDH5 expression as an independent prognostic factor (p < 0.0001). Immunohistochemical double staining experiments revealed LDH5 expression by cancer cells in association with glucose uptake (GLUT-1), proliferation (Ki-67), and hypoxia (HIF-1α). LDH5 specificity was confirmed by western blot and RT-PCR analysis. For the first time, this study provides evidence that LDH5 expression in OSCC might be associated with tumour formation and metastasis in a large patient cohort. Therefore, adjuvant therapies targeting glucose metabolism might be promising for therapy of OSCC.





Similar content being viewed by others
Abbreviations
- OSCC:
-
Oral squamous cell carcinoma
- HE:
-
Hematoxylin and eosin
- ROC:
-
Receiver operating characteristics analysis
- AUC:
-
Area under the curve analysis
References
Grimm M (2012) Prognostic value of clinicopathological parameters and outcome in 484 patients with oral squamous cell carcinoma: microvascular invasion (V+) is an independent prognostic factor for OSCC. Clin Transl Oncol 14(11):870–880. doi:10.1007/s12094-012-0867-2
Perez-Sayans M, Suarez-Penaranda JM, Pilar GD, Barros-Angueira F, Gandara-Rey JM, Garcia-Garcia A (2011) Hypoxia-inducible factors in OSCC. Cancer Lett 313(1):1–8. doi:10.1016/j.canlet.2011.08.017
Lothaire P, de Azambuja E, Dequanter D, Lalami Y, Sotiriou C, Andry G, Castro G Jr, Awada A (2006) Molecular markers of head and neck squamous cell carcinoma: promising signs in need of prospective evaluation. Head Neck 28(3):256–269. doi:10.1002/hed.20326
Warburg O, Posener K, Negelein E (1924) Über den Stoffwechsel der Carcinomzelle. Biochem Z 152:309–344
Holbrook J, Liljas A, Steindel S, Rossman M (1975) Lactate dehydrogenase. In: Boyer PD (ed) The Enzymes, vol 11, 3rd edn. Academic Press, New York, pp 191–292
De Milito A, Fais S (2005) Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol 1(6):779–786. doi:10.2217/14796694.1.6.779
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7(1):11–20. doi:10.1016/j.cmet.2007.10.002
Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A (1996) Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271(51):32529–32537
Vaupel P (2008) Hypoxia and aggressive tumor phenotype: implications for therapy and prognosis. Oncologist 13(Suppl 3):21–26. doi:10.1634/theoncologist.13-S3-21
Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, Bartenstein P, Wagner W, Whiteside TL (2003) Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer 97(4):1015–1024. doi:10.1002/cncr.11159
Eckert AW, Lautner MH, Schutze A, Bolte K, Bache M, Kappler M, Schubert J, Taubert H, Bilkenroth U (2010) Co-expression of Hif1alpha and CAIX is associated with poor prognosis in oral squamous cell carcinoma patients. J Oral Pathol Med 39(4):313–317. doi:10.1111/j.1600-0714.2009.00829.x
Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, Harris AL (2003) Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer 89(5):877–885. doi:10.1038/sj.bjc.6601205
Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Trarbach T, Folprecht G, Shi MM, Lebwohl D, Jalava T, Laurent D, Meinhardt G, Harris AL (2011) Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin Cancer Res 17(14):4892–4900. doi:10.1158/1078-0432.CCR-10-2918
Kayser G, Kassem A, Sienel W, Schulte-Uentrop L, Mattern D, Aumann K, Stickeler E, Werner M, Passlick B, zur Hausen A (2010) Lactate-dehydrogenase 5 is overexpressed in non-small cell lung cancer and correlates with the expression of the transketolase-like protein 1. Diagn Pathol 5:22. doi:10.1186/1746-1596-5-22
Kolev Y, Uetake H, Takagi Y, Sugihara K (2008) Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol 15(8):2336–2344. doi:10.1245/s10434-008-9955-5
Giatromanolaki A, Sivridis E, Gatter KC, Turley H, Harris AL, Koukourakis MI (2006) Lactate dehydrogenase 5 (LDH-5) expression in endometrial cancer relates to the activated VEGF/VEGFR2(KDR) pathway and prognosis. Gynecol Oncol 103(3):912–918. doi:10.1016/j.ygyno.2006.05.043
Koukourakis MI, Giatromanolaki A, Winter S, Leek R, Sivridis E, Harris AL (2009) Lactate dehydrogenase 5 expression in squamous cell head and neck cancer relates to prognosis following radical or postoperative radiotherapy. Oncology 77(5):285–292. doi:10.1159/000259260
Zhuang L, Scolyer RA, Murali R, McCarthy SW, Zhang XD, Thompson JF, Hersey P (2010) Lactate dehydrogenase 5 expression in melanoma increases with disease progression and is associated with expression of Bcl-XL and Mcl-1, but not Bcl-2 proteins. Mod Pathol 23(1):45–53. doi:10.1038/modpathol.2009.129
Giatromanolaki A, Koukourakis MI, Pezzella F, Sivridis E, Turley H, Harris AL, Gatter KC (2008) Lactate dehydrogenase 5 expression in non-Hodgkin B-cell lymphomas is associated with hypoxia regulated proteins. Leuk Lymphoma 49(11):2181–2186. doi:10.1080/10428190802450629
Sobin LH, Ch W (2010) UICC. TNM classification of malignant tumors, 7th edn. Springer Verlag, Berlin
Hamilton SR, Aaltonen LA (2000) Pathology and genetics. Tumours of the digestive system, 3rd edn. IARC Press, Lyon
von Rahden BH, Kircher S, Kafka M, Stuermer L, Reiber C, Gattenlohner S, Germer CT, Grimm M (2010) Glucocorticoid-induced TNFR family-related receptor (GITR)-expression in tumor infiltrating leucocytes (TILs) is associated with the pathogenesis of esophageal adenocarcinomas with and without Barrett’s mucosa. Cancer Biomark 7(6):285–294. doi:10.3233/CBM-2010-0192
Piret JP, Mottet D, Raes M, Michiels C (2002) CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Ann NY Acad Sci 973:443–447
Grimm M, Lazariotou M, Kircher S, Stuermer L, Reiber C, Hofelmayr A, Gattenlohner S, Otto C, Germer CT, von Rahden BH (2010) MMP-1 is a (pre-)invasive factor in Barrett-associated esophageal adenocarcinomas and is associated with positive lymph node status. J Transl Med 8:99. doi:10.1186/1479-5876-8-99
Alexander D, Hoffmann J, Munz A, Friedrich B, Geis-Gerstorfer J, Reinert S (2008) Analysis of OPLA scaffolds for bone engineering constructs using human jaw periosteal cells. J Mater Sci Mater Med 19(3):965–974. doi:10.1007/s10856-007-3351-8
Alexander D, Schafer F, Olbrich M, Friedrich B, Buhring HJ, Hoffmann J, Reinert S (2010) MSCA-1/TNAP selection of human jaw periosteal cells improves their mineralization capacity. Cell Physiol Biochem 26(6):1073–1080. doi:10.1159/000323985
Zweig MH, Campbell G (1993) Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39(4):561–577
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 75:457–487
Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50(3):163–170
Cox DR (1972) Regression models and life tables. J R Stat Soc 34:1987–2001
Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL (2006) Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway–a report of the Tumour Angiogenesis Research Group. J Clin Oncol 24(26):4301–4308. doi:10.1200/JCO.2006.05.9501
Huang Q, Yu GP, McCormick SA, Mo J, Datta B, Mahimkar M, Lazarus P, Schaffer AA, Desper R, Schantz SP (2002) Genetic differences detected by comparative genomic hybridization in head and neck squamous cell carcinomas from different tumor sites: construction of oncogenetic trees for tumor progression. Genes Chromosomes Cancer 34(2):224–233. doi:10.1002/gcc.10062
Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV (2010) Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA 107(5):2037–2042. doi:10.1073/pnas.0914433107
Langhammer S, Najjar M, Hess-Stumpp H, Thierauch KH (2011) LDH-A influences hypoxia-inducible factor 1alpha (HIF1 alpha) and is critical for growth of HT29 colon carcinoma cells in vivo. Target Oncol 6(3):155–162. doi:10.1007/s11523-011-0184-7
Xie H, Valera VA, Merino MJ, Amato AM, Signoretti S, Linehan WM, Sukhatme VP, Seth P (2009) LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther 8(3):626–635. doi:10.1158/1535-7163.MCT-08-1049
Zhang Y, Zhang X, Wang X, Gan L, Yu G, Chen Y, Liu K, Li P, Pan J, Wang J, Qin S (2012) Inhibition of LDH-A by lentivirus-mediated small interfering RNA suppresses intestinal-type gastric cancer tumorigenicity through the downregulation of Oct4. Cancer Lett 321(1):45–54. doi:10.1016/j.canlet.2012.03.013
Wang ZY, Loo TY, Shen JG, Wang N, Wang DM, Yang DP, Mo SL, Guan XY, Chen JP (2012) LDH-A silencing suppresses breast cancer tumorigenicity through induction of oxidative stress mediated mitochondrial pathway apoptosis. Breast Cancer Res Treat 131(3):791–800. doi:10.1007/s10549-011-1466-6
Lukacova S, Sorensen BS, Alsner J, Overgaard J, Horsman MR (2008) The impact of hypoxia on the activity of lactate dehydrogenase in two different pre-clinical tumour models. Acta Oncol 47(5):941–947. doi:10.1080/02841860701644086
Wigfield SM, Winter SC, Giatromanolaki A, Taylor J, Koukourakis ML, Harris AL (2008) PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer 98(12):1975–1984. doi:10.1038/sj.bjc.6604356
Quennet V, Yaromina A, Zips D, Rosner A, Walenta S, Baumann M, Mueller-Klieser W (2006) Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother Oncol 81(2):130–135. doi:10.1016/j.radonc.2006.08.012
Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, Mueller-Klieser W (2001) Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys 51(2):349–353
Wangsa D, Ryott M, Avall-Lundqvist E, Petersson F, Elmberger G, Luo J, Ried T, Auer G, Munck-Wikland E (2008) Ki-67 expression predicts locoregional recurrence in stage I oral tongue carcinoma. Br J Cancer 99(7):1121–1128. doi:10.1038/sj.bjc.6604633
Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A (2005) Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem 280(51):41928–41939. doi:10.1074/jbc.M508718200
Van Cutsem E, Bajetta E, Valle J, Kohne CH, Hecht JR, Moore M, Germond C, Berg W, Chen BL, Jalava T, Lebwohl D, Meinhardt G, Laurent D, Lin E (2011) Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncolc 29(15):2004–2010. doi:10.1200/JCO.2010.29.5436
Scartozzi M, Giampieri R, Maccaroni E, Del Prete M, Faloppi L, Bianconi M, Galizia E, Loretelli C, Belvederesi L, Bittoni A, Cascinu S (2012) Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br J Cancer 106(5):799–804. doi:10.1038/bjc.2012.17
Scartozzi M, Faloppi L, Bianconi M, Giampieri R, Maccaroni E, Bittoni A, Del Prete M, Loretelli C, Belvederesi L, Svegliati Baroni G, Cascinu S (2012) The role of LDH serum levels in predicting global outcome in HCC patients undergoing TACE: implications for clinical management. PLoS One 7(3):e32653. doi:10.1371/journal.pone.0032653
Nebeling LC, Miraldi F, Shurin SB, Lerner E (1995) Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr 14(2):202–208
Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, Voelker HU, Thiede A, Coy JF (2008) Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer 8:122. doi:10.1186/1471-2407-8-122
Seyfried TN, Kiebish M, Mukherjee P, Marsh J (2008) Targeting energy metabolism in brain cancer with calorically restricted ketogenic diets. Epilepsia 49(Suppl 8):114–116. doi:10.1111/j.1528-1167.2008.01853.x
Seyfried BT, Kiebish M, Marsh J, Mukherjee P (2009) Targeting energy metabolism in brain cancer through calorie restriction and the ketogenic diet. J Cancer Res Ther 5(Suppl 1):S7–S15. doi:10.4103/0973-1482.55134
Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN (2007) The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond) 4:5. doi:10.1186/1743-7075-4-5
Schmidt M, Pfetzer N, Schwab M, Strauss I, Kammerer U (2011) Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab 8(1):54. doi:10.1186/1743-7075-8-54
Fine EJ, Segal-Isaacson CJ, Feinman RD, Herszkopf S, Romano MC, Tomuta N, Bontempo AF, Negassa A, Sparano JA (2012) Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition 28(10):1028–1035. doi:10.1016/j.nut.2012.05.001
Severino P, Alvares AM, Michaluart P Jr, Okamoto OK, Nunes FD, Moreira-Filho CA, Tajara EH (2008) Global gene expression profiling of oral cavity cancers suggests molecular heterogeneity within anatomic subsites. BMC Res Notes 1:113. doi:10.1186/1756-0500-1-113
Acknowledgments
We thank Julia Fertinger, Mohammed Saleh, and Besher Shokri for their technical assistance.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grimm, M., Alexander, D., Munz, A. et al. Increased LDH5 expression is associated with lymph node metastasis and outcome in oral squamous cell carcinoma. Clin Exp Metastasis 30, 529–540 (2013). https://doi.org/10.1007/s10585-012-9557-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-012-9557-2