Abstract
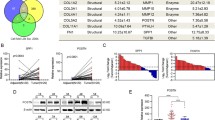
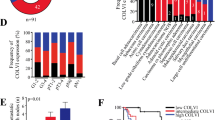
Recently, the tumor microenvironment is increasingly recognized as playing an important role in cancer proliferation, invasion, and metastasis. To screen stroma-associated proteins involved in nasopharyngeal carcinoma (NPC) carcinogenesis, laser capture microdissection (LCM) and quantitative proteomic analysis were employed to assess different protein expression of the stroma between NPC and normal nasopharyngeal mucosa (NNM). In this study, periostin was identified to be significantly up-regulated in NPC stroma compared with NNM stroma and the result was further confirmed by Western blotting. Immunohistochemistry showed that over-expression of periostin was frequently observed in the stroma of NPC and matched lymph node metastases (LNM) compared with the stroma of NNM. Statistical analysis showed over-expression of periostin was significantly associated with advanced clinical stage (P < 0.001) and lymph node metastasis (P < 0.001) and decreased overall survival (P < 0.001) in NPC. Cox regression analysis indicated over-expression of periostin was an independent prognostic factor. Furthermore, ectopic expression of periostin was used to examine its effect on invasiveness of NPC cell in vitro and the result showed that periostin was able to promote invasiveness of NPC cell. In conclusion, periostin expression is correlated with tumor stage, lymph node metastasis, and patient survival. Periostin is a potential biomarker for the differentiation and prognosis of NPC, and it might play an important role in the progression of NPC.





Similar content being viewed by others
Abbreviations
- NPC:
-
Nasopharyngeal carcinoma
- NNM:
-
Normal nasopharyngeal mucosa
- LCM:
-
Laser capture microdissection
- LNM:
-
Lymph node metastases
- TS:
-
Tumor stroma
- NS:
-
Normal stroma
- 2D-DIGE:
-
Two-dimensional difference gel electrophoresis
- MS:
-
Mass spectrometry
References
Albini A (2008) Tumor microenvironment, a dangerous society leading to cancer metastasis. From mechanisms to therapy and prevention. Cancer Metastasis Rev 27(1):3–4
Zigrino P, Loffek S, Mauch C (2005) Tumor–stroma interactions: their role in the control of tumor cell invasion. Biochimie 87(3–4):321–328
Sung SY et al (2007) Tumor microenvironment promotes cancer progression, metastasis, and therapeutic resistance. Curr Probl Cancer 31(2):36–100
Hiscox S, Barrett-Lee P, Nicholson RI (2011) Therapeutic targeting of tumor-stroma interactions. Expert Opin Ther Targets 15(5):609–621
Marsh D et al (2011) Stromal features are predictive of disease mortality in oral cancer patients. J Pathol 223(4):470–481
Wernert N et al (2001) Presence of genetic alterations in microdissected stroma of human colon and breast cancers. Anticancer Res 21(4A):2259–2264
Liotta LA, Kohn EC (2001) The microenvironment of the tumour–host interface. Nature 411(6835):375–379
Bissell MJ, Radisky D (2001) Putting tumours in context. Nat Rev Cancer 1(1):46–54
Yu MC, Yuan JM (2002) Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol 12(6):421–429
Choi PH et al (2011) Nasopharyngeal carcinoma: genetic changes, Epstein-Barr virus infection, or both. A clinical and molecular study of 36 patients. Cancer 72(10):2873–2878
Ali H, Al-Sarraf M (1999) Nasopharyngeal cancer. Hematol Oncol Clin North Am 13(4):837–847
Vokes EE, Liebowitz DN, Weichselbaum RR (1997) Nasopharyngeal carcinoma. Lancet 350(9084):1087–1091
Ahmad A, Stefani S (1986) Distant metastases of nasopharyngeal carcinoma: a study of 256 male patients. J Surg Oncol 33(3):194–197
Cheng AL et al (2008) Identification of novel nasopharyngeal carcinoma biomarkers by laser capture microdissection and proteomic analysis. Clin Cancer Res 14(2):435–445
Qian CN et al (2002) Met protein expression level correlates with survival in patients with late-stage nasopharyngeal carcinoma. Cancer Res 62(2):589–596
Hwang CF et al (2010) Fibulin-3 is associated with tumour progression and a poor prognosis in nasopharyngeal carcinomas and inhibits cell migration and invasion via suppressed AKT activity. J Pathol 222(4):367–379
Wang S et al (2010) TFPI-2 is a putative tumor suppressor gene frequently inactivated by promoter hypermethylation in nasopharyngeal carcinoma. BMC Cancer 10:617
Buettner M et al (2007) Expression of RANTES and MCP-1 in epithelial cells is regulated via LMP1 and CD40. Int J Cancer 121(12):2703–2710
Cho WC et al (2004) Identification of serum amyloid a protein as a potentially useful biomarker to monitor relapse of nasopharyngeal cancer by serum proteomic profiling. Clin Cancer Res 10(1 Pt 1):43–52
Zhou Y et al (2008) Identification of candidate molecular markers of nasopharyngeal carcinoma by microarray analysis of subtracted cDNA libraries constructed by suppression subtractive hybridization. Eur J Cancer Prev 17(6):561–571
Friedman DB et al (2004) Proteome analysis of human colon cancer by two-dimensional difference gel electrophoresis and mass spectrometry. Proteomics 4(3):793–811
Kakisaka T et al (2007) Plasma proteomics of pancreatic cancer patients by multi-dimensional liquid chromatography and two-dimensional difference gel electrophoresis (2D-DIGE): up-regulation of leucine-rich alpha-2-glycoprotein in pancreatic cancer. J Chromatogr B Analyt Technol Biomed Life Sci 852(1–2):257–267
Horiuchi K et al (1999) Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14(7):1239–1249
Litvin J et al (2005) Periostin family of proteins: therapeutic targets for heart disease. Anat Rec A Discov Mol Cell Evol Biol 287(2):1205–1212
Takeshita S et al (1993) Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J 294(Pt 1):271–278
Gillan L et al (2002) Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res 62(18):5358–5364
Michaylira CZ et al (2010) Periostin, a cell adhesion molecule, facilitates invasion in the tumor microenvironment and annotates a novel tumor-invasive signature in esophageal cancer. Cancer Res 70(13):5281–5292
Ruan K, Bao S, Ouyang G (2009) The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci 66(14):2219–2230
Erkan M et al (2007) Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology 132(4):1447–1464
Baril P et al (2007) Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene 26(14):2082–2094
Bao S et al (2004) Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 5(4):329–339
Kanno A et al (2008) Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer 122(12):2707–2718
Tischler V et al (2010) Periostin is up-regulated in high grade and high stage prostate cancer. BMC Cancer 10:273
Kudo Y et al (2006) Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res 66(14):6928–6935
Puppin C et al (2008) High periostin expression correlates with aggressiveness in papillary thyroid carcinomas. J Endocrinol 197(2):401–408
Soltermann A et al (2008) Prognostic significance of epithelial-mesenchymal and mesenchymal-epithelial transition protein expression in non-small cell lung cancer. Clin Cancer Res 14(22):7430–7437
Puglisi F et al (2008) Expression of periostin in human breast cancer. J Clin Pathol 61(4):494–498
Song LB et al (2002) Molecular mechanisms of tumorigenesis and metastasis in nasopharyngeal carcinoma cell sublines. Ai Zheng 21(2):158–162
Cheung HW et al (2005) Mitotic arrest deficient 2 expression induces chemosensitization to a DNA-damaging agent, cisplatin, in nasopharyngeal carcinoma cells. Cancer Res 65(4):1450–1458
Cheng AL et al (2008) Identificating cathepsin D as a biomarker for differentiation and prognosis of nasopharyngeal carcinoma by laser capture microdissection and proteomic analysis. J Proteome Res 7(6):2415–2426
Hermani A et al (2005) Calcium-binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clin Cancer Res 11(14):5146–5152
Li MX et al (2009) Quantitative proteomic analysis of differential proteins in the stroma of nasopharyngeal carcinoma and normal nasopharyngeal epithelial tissue. J Cell Biochem 106(4):570–579
Thompson CC et al (2007) Pancreatic cancer cells overexpress gelsolin family-capping proteins, which contribute to their cell motility. Gut 56(1):95–106
Nomura H et al (2008) Clinical significance of gelsolin-like actin-capping protein expression in oral carcinogenesis: an immunohistochemical study of premalignant and malignant lesions of the oral cavity. BMC Cancer 8:39
Li MX et al (2010) Proteomic analysis of the stroma-related proteins in nasopharyngeal carcinoma and normal nasopharyngeal epithelial tissues. Med Oncol 27(1):134–144
Kim SH et al (2011) Nuclear localization of Nm23-H1 in head and neck squamous cell carcinoma is associated with radiation resistance. Cancer 117(9):1864–1873
Wu M et al (2009) Signaling transduction network mediated by tumor suppressor/susceptibility genes in NPC. Curr Genomics 10(4):216–222
Kikuchi Y et al (2008) Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem 56(8):753–764
Chen ZG (2007) Exploration of metastasis-related proteins as biomarkers and therapeutic targets in the treatment of head and neck cancer. Curr Cancer Drug Targets 7(7):613–622
Siriwardena BS et al (2006) Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br J Cancer 95(10):1396–1403
Acknowledgments
This research was supported by grants from National Natural Sciences Foundation of China (Grant No. 81072199, 30973289), Scientific Research Foundation for Dr. of University of South China (2010XQD38), National Basic Research Program of China (2011CB910704), Science and Technology Research Program of Hunan Province in China (2011TT2015). We gratefully acknowledge Professor Rong Shao (University of Massachusetts, USA) for providing pCMV-neo-periostin plasmid.
Conflict of interest
No conflicts of interest are declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M., Li, C., Li, D. et al. Periostin, a stroma-associated protein, correlates with tumor invasiveness and progression in nasopharyngeal carcinoma. Clin Exp Metastasis 29, 865–877 (2012). https://doi.org/10.1007/s10585-012-9465-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-012-9465-5