Abstract
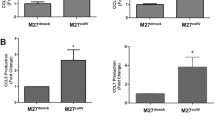
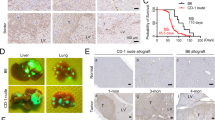
In many different tumor entities, increased expression of tissue inhibitor of metalloproteinases-1 (Timp-1) is associated with poor prognosis. We previously reported in mouse models that elevated systemic levels of Timp-1 induce a gene expression signature in the liver microenvironment increasing the susceptibility of this organ to tumor cells. This host effect was dependent on increased activity of the hepatocyte growth factor (Hgf)/hepatocyte growth factor receptor (Met) signaling pathway. In a recent study we showed that Met signaling is regulated by Timp-1 as it inhibits the Met sheddase A disintegrin and metalloproteinase-10 (Adam-10). The aim of the present study was to elucidate whether the metastatic potential of tumor cells benefits from autocrine Timp-1 as well and involves Adam-10 and Met signaling. In a syngeneic murine model of experimental liver metastasis Timp-1 expression and Met signaling were localized within metastatic colonies and expressed by tumor cells. Knock down of tumor cell Timp-1 suppressed Met signaling in metastases and inhibited metastasis formation and tumor cell-scattering in the liver. In vitro, knock down of tumor cell Timp-1 prevented Hgf-induced Met phosphorylation. Consequently, knock down of Met sheddase Adam-10 triggered auto-phosphorylation and responsiveness to Hgf. Accordingly, Adam-10 knock down increased Met phosphorylation in metastatic foci and induced tumor cell scattering into the surrounding liver parenchyma. In conclusion, these findings show that tumor cell-derived Timp-1 acts as a positive regulator of the metastatic potential and support the concept that proteases and their natural inhibitors, as members of the protease web, are major players of signaling during normal homeostasis and disease.





Similar content being viewed by others
Abbreviations
- DAPI:
-
4′,6-Diamidine-2-phenylindole
- Hgf:
-
Hepatocyte growth factor
- i.v.:
-
Intraveneous
- Met:
-
Hepatocyte growth factor receptor
- MMP:
-
Matrix metalloproteinase
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- rec:
-
Recombinant
- SF:
-
Scatter factor
- sh:
-
Small hairpin
- TBS:
-
2-Amino-2-(hydroxymethyl)-propane-1,3-diole-buffered saline
- Timp:
-
Tissue inhibitor of metalloproteinases
- X-Gal:
-
5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside
References
Terpos E, Dimopoulos MA, Shrivastava V et al (2010) High levels of serum TIMP-1 correlate with advanced disease and predict for poor survival in patients with multiple myeloma treated with novel agents. Leuk Res 34(3):399–402
Aaberg-Jessen C, Christensen K, Offenberg H et al (2009) Low expression of tissue inhibitor of metalloproteinases-1 (TIMP-1) in glioblastoma predicts longer patient survival. J Neurooncol 95(1):117–128
Scrideli CA, Cortez MA, Yunes JA et al (2010) mRNA expression of matrix metalloproteinases (MMPs) 2 and 9 and tissue inhibitor of matrix metalloproteinases (TIMPs) 1 and 2 in childhood acute lymphoblastic leukemia: potential role of TIMP1 as an adverse prognostic factor. Leuk Res 34(1):32–37
Rauvala M, Puistola U, Turpeenniemi-Hujanen T (2005) Gelatinases and their tissue inhibitors in ovarian tumors; TIMP-1 is a predictive as well as a prognostic factor. Gynecol Oncol 99(3):656–663
Chirco R, Liu XW, Jung KK et al (2006) Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 25(1):99–113
Mook OR, Frederiks WM, Van Noorden CJ (2004) The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta 1705(2):69–89
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2(3):161–174
Coussens LM, Fingleton B, Matrisian LM (2002) Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295(5564):2387–2392
Deryugina EI, Quigley JP (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25(1):9–34
Gutierrez-Fernandez A, Fueyo A, Folgueras AR et al (2008) Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res 68(8):2755–2763
Garg P, Sarma D, Jeppsson S et al (2010) Matrix metalloproteinase-9 functions as a tumor suppressor in colitis-associated cancer. Cancer Res 70(2):792–801
Krüger A et al (2001) Hydroxamate-type matrix metalloproteinase inhibitor batimastat promotes liver metastasis. Cancer Res 61(4):1272–1275
Schelter F, Halbgewachs B, Baumler P et al (2011) Tissue inhibitor of metalloproteinases-1-induced scattered liver metastasis is mediated by hypoxia-inducible factor-1alpha. Clin Exp Metastasis 28(2):91–99
Kopitz C, Gerg M, Bandapalli OR et al (2007) Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res 67(18):8615–8623
Krüger A (2009) Functional genetic mouse models: promising tools for investigation of the proteolytic internet. Biol Chem 390(2):91–97
Overall CM, Kleifeld O (2006) Tumour microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer 6(3):227–239
Krüger A, Kates RE, Edwards DR (2010) Avoiding spam in the proteolytic internet: future strategies for anti-metastatic MMP inhibition. Biochim Biophys Acta 1803(1):95–102
Gerg M, Kopitz C, Schaten S et al (2008) Distinct functionality of tumor cell-derived gelatinases during formation of liver metastases. Mol Cancer Res 6(3):341–351
Schelter F, Gerg M, Halbgewachs B et al (2010) Identification of a survival-independent metastasis-enhancing role of hypoxia-inducible factor-1alpha with a hypoxia-tolerant tumor cell line. J Biol Chem 285(34):26182–26189
Naldini L, Weidner KM, Vigna E et al (1991) Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J 10(10):2867–2878
Boccaccio C, Comoglio PM (2006) Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer 6(8):637–645
Trusolino L, Bertotti A, Comoglio PM (2010) MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 11(12):834–848
Comoglio PM, Giordano S, Trusolino L (2008) Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 7(6):504–516
Murphy G (2008) The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer 8(12):929–941
Amour A, Knight CG, Webster A et al (2000) The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett 473(3):275–279
Schelter F, Kobuch J, Moss ML et al (2010) A disintegrin and metalloproteinase-10 (ADAM-10) mediates DN30 antibody-induced shedding of the met surface receptor. J Biol Chem 285(34):26335–26340
Schirrmeister W, Gnad T, Wex T et al (2009) Ectodomain shedding of E-cadherin and c-Met is induced by Helicobacter pylori infection. Exp Cell Res 315(20):3500–3508
Koshariya M, Jagad RB, Kawamoto J et al (2007) An update and our experience with metastatic liver disease. Hepatogastroenterology 54(80):2232–2239
Sporn MB (1996) The war on cancer. Lancet 347(9012):1377–1381
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Krüger A, Schirrmacher V, von Hoegen P (1994) Scattered micrometastases visualized at the single-cell level: detection and re-isolation of lacZ-labeled metastasized lymphoma cells. Int J Cancer 58(2):275–284
Soneoka Y, Cannon PM, Ramsdale EE et al (1995) A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res 23(4):628–633
Krüger A, Schirrmacher V, Khokha R (1998) The bacterial lacZ gene: an important tool for metastasis research and evaluation of new cancer therapies. Cancer Metastasis Rev 17(3):285–294
Schrötzlmair F, Kopitz C, Halbgewachs B et al (2010) Tissue inhibitor of metalloproteinases-1-induced scattered liver metastasis is mediated by host-derived urokinase-type plasminogen activator. J Cell Mol Med 14(12):2760–2770
Brand K et al (2000) Treatment of colorectal liver metastases by adenoviral transfer of tissue inhibitor of metalloproteinases-2 into the liver tissue. Cancer Res 60(20):5723–5730
Baker AH, Edwards DR, Murphy G (2002) Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 115(Pt 19):3719–3727
Bajou K et al (1998) Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med 4(8):923–928
Krüger A, Fata JE, Khokha R (1997) Altered tumor growth and metastasis of a T-cell lymphoma in Timp-1 transgenic mice. Blood 90(5):1993–2000
Elezkurtaj S, Kopitz C, Baker AH et al (2004) Adenovirus-mediated overexpression of tissue inhibitor of metalloproteinases-1 in the liver: efficient protection against T-cell lymphoma and colon carcinoma metastasis. J Gene Med 6(11):1228–1237
Brew K, Nagase H (2010) The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 1803(1):55–71
Foveau B et al (2009) Down-regulation of the met receptor tyrosine kinase by presenilin-dependent regulated intramembrane proteolysis. Mol Biol Cell 20(9):2495–2507
Amour A et al (1998) TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett 435(1):39–44
Moss ML, Stoeck A, Yan W et al (2008) ADAM10 as a target for anti-cancer therapy. Curr Pharm Biotechnol 9(1):2–8
Würtz SO, Schrohl AS, Sorensen NM et al (2005) Tissue inhibitor of metalloproteinases-1 in breast cancer. Endocr Relat Cancer 12(2):215–227
Acknowledgments
We thank Katja Honert and Stefan Grötzinger (all from Institut für Experimentelle Onkologie und Therapieforschung des Klinikums rechts der Isar, Technische Universität München, Munich, Germany) for their expert technical assistance and Gillian Murphy for providing the anti-Timp-1 antibody. For financial support, the authors thank the European Union Research Framework Programme 7, project HEALTH-2007-201279/Microenvimet (to Achim Krüger, Carla Boccaccio and Paolo Comoglio).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schelter, F., Grandl, M., Seubert, B. et al. Tumor cell-derived Timp-1 is necessary for maintaining metastasis-promoting Met-signaling via inhibition of Adam-10. Clin Exp Metastasis 28, 793–802 (2011). https://doi.org/10.1007/s10585-011-9410-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-011-9410-z