Abstract
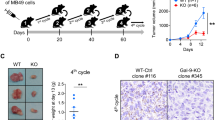
Galectin-3, a β galactoside–binding lectin, plays an important role in the processes relevant to tumorigenesis such as malignant cell transformation, invasion and metastasis. We have investigated whether deletion of Galectin-3 in the host affects the metastasis of B16F1 malignant melanoma. Galectin-3-deficient (Gal-3−/−) mice are more resistant to metastatic malignant melanoma as evaluated by number and size of metastatic colonies in the lung. In vitro assays showed lower number of attached malignant cells in the tissue section derived from Gal-3−/− mice. Furthermore, lack of Galectin-3 correlates with higher serum levels of IFN-γ and IL-17 in tumor bearing hosts. Interestingly, spleens of Gal-3−/− mice have lower number of Foxp3+ T cells after injection of B16F1 melanoma cells. Finally, we found that while CD8+ T cell and adherent cell cytotoxicity were similar, there was greater cytotoxic activity of splenic NK cells of Gal-3−/− mice compared with “wild-type” (Gal-3+/+) mice. Despite the reduction in total number of CD3ε−NK1.1+, Gal-3−/− mice constitutively have a significantly higher percentage of effective cytotoxic CD27highCD11bhigh NK cells as well as the percentage of immature CD27highCD11blow NK cells. In contrast, CD27lowCD11bhigh less functionally exhausted NK cells and NK cells bearing inhibitory KLRG1 receptor were more numerous in Gal-3+/+ mice. It appears that lack of Galectin-3 affects tumor metastasis by at least two independent mechanisms: by a decrease in binding of melanoma cells onto target tissue and by enhanced NK-mediated anti-tumor response suggesting that Galectin-3 may be considered as therapeutic target.






Similar content being viewed by others
Abbreviations
- B16F1:
-
Murine skin melanoma cell line
- Gal-3:
-
Galectin-3
- IFN-γ:
-
Interferon-gamma
- IL-17:
-
Interleukin-17
- IL-4:
-
Interleukin-4
- KLRG1:
-
Killer cell lectin-like receptor G1
- NK cells:
-
Natural killer cells
- TNF-α:
-
Tumor necrosis-alpha
References
Sato S, Hughes RCJ (1994) Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem 269:4424–4430
Moutsatsos IK, Wade M, Schindler M et al (1987) Endogenous lectins from cultured cells: nuclear localization of carbohydrate binding protein 35 in proliferating 3T3 fibroblasts. Proc Natl Acad Sci USA 84:6452–6456
Perillo NL, Marcus ME, Baum LG (1998) Galectins: Versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med 76:402–412
Dumic J, Dabelic S, Flögel M (2006) Galectin-3: an open-ended story. Biochim Biophys Acta 1760:616–635
Liu FT, Rabinovich GA (2005) Galectins as modulators of tumour progression. Nat Rev Cancer 5:29–41
Yang RY, Rabinovich GA, Liu FT (2008) Galectins: structure, function and therapeutic potential. Expert Rev Mol Med 10:e17
Yilmaz M, Christofori G, Lehembre F (2007) Distinct mechanisms of tumor invasion and metastasis. Trends Mol Med 13(12):535–541
Nicolson GL, Winkelhake JL (1975) Organ specificity of blood-borne tumour metastasis determined by cell adhesion? Nature 255:230–232
Miles FL, Pruitt FL, van-Golen KL et al (2008) Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis 25:305–324
Zhao Q, Guo X, Nash GB et al (2009) Circulating Galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res 69(17):6799–6806
Takenaka Y, Fukumori T, Raz A (2004) Galectin-3 and metastasis. Glycoconj J 19:543–549
Glinsky VV, Glinsky GV, Rittenhouse-Olson K et al (2001) The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res 61:4851–4857
Krishnan V, Bane SM, Kawle PD et al (2005) Altered melanoma cell surface glycosylation mediates organ specific adhesion and via lectin receptors on the lung vascular endothelium. Clin Exp Metastasis 22:11–24
Danguy A, Camby I, Kiss R (2002) Galectins and cancers. Biochim Biopys Acta 1572:285–293
Prieto VG, Mourad-Zeidan AA, Melnikova V et al (2006) Galectin-3 expression is associated with tumor progression and pattern of sun exposure in melanoma. Clin Cancer Res 12:6709–6715
Iurisci I, Tinari N, Natoli C et al (2000) Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res 6:1389–1393
Vereecken P, Zouaoui Boudjeltia K, Debray C et al (2006) High serum galectin-3 in advanced melanoma: preliminary results. Clin Exp Dermatol 31:105–109
Ochieng J, Warfield P, Green-Jarvis B et al (1999) Galectin-3 regulates the adhesive interaction between breast carcinoma cells and elastin. J Cell Biochem 75:505–514
Le Marer N, Hughes RC (1996) Effects of the carbohydrate-binding protein galectin-3 on the invasiveness of human breast carcinoma cells. J Cell Physiol 168:51–58
Thijssen VL, Poirier F, Baum LG et al (2007) Galectins in the tumor endothelium: opportunities for combined cancer therapy. Blood 110(8):2819–2827
Smyth MJ, Hayakawa Y, Takeda K et al (2002) New aspects of natural-killer cell surveillance and therapy of cancer. Nat Rev Cancer 2:850–861
Yu P, Fu YX (2006) Tumor-infiltrating T lymphcytes: friends or foels? Lab Invest 86:231–245
Zubieta MR, Furman D, Barrio M et al (2006) Galectin-3 expression correlates with apoptosis of tumor-associated lymphocytes in human melanoma biopsies. Am J Pathol 168:1666–1675
Peng W, Wang HY, Miyahara Y et al (2008) Tumor-associated galectin-3 modulates the function of tumor-reactive T cells. Cancer Res 68:7228–7236
Lee VH, Lee AB, Phillips EB et al (1998) Spatio-temporal pattern for expression of galectin-3 in the murine utero-placental complex: Evidence for differential regulation. Biol Reprod 58:1277–1282
Crider-Pirkle S, Billingsley P, Faust C et al (2002) Cubilin, a binding partner for galectin-3 in the murine utero-placental complex. J Biol Chem 277(18):15904–15912
Grundy MA, Zhang T, Sentman CL (2007) NK cells rapidly remove B16F10 tumor cells in a perforin and interferon-gamma independent manner in vivo. Cancer Immunol Immunother 56:1153–1161
Street SE, Cretney E, Smyth MJ (2001) Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 97:192–197
Hsu DK, Yang RY, Pan Z et al (1994) Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol 156:1073–1083
Edward M, Gold JA, McKie MR (1989) Modulation of melanoma cell adhesion to basement membrane components by retinoic acid. J Cell Sci 93:155–161
Netland PA, Zetter BR (1984) Organ-specific adhesion of metastatic tumor cells in vitro. Science 224:1113–1135
Janjic BM, Lu G, Pimenov A et al (2002) Innate direct anticancer effector function of human immature dendritic cells. I. Involvement of an apoptosis-inducing pathway. J Immunol 168(4):1823–1830
Richards J, McNally B, Fang X et al (2008) Tumor growth decreases NK and B cells as well as common lymphoid progenitor. PLoS One 3(9):e3180
Calof AL, Campanero MR, O’Rear JJ et al (1994) Domain-specific activation of neuronal migration and neurite outgrowth- promoting activities of laminin. Neuron 13(1):117–130
Raz A, Zhu DG, Hogan V et al (1990) Evidence for the role of 34 kDa galactoside-binding lectin in transformation and metastasis. Int J Cancer 46:871–877
Nakahara S, Oka N, Raz A (2005) On the role of galectin-3 in cancer apoptosis. Apoptosis 10:267–275
Fukumori T, Oka N, Takenaka Y et al (2006) Galectin-3 regulates mitochondrial stability and antiapoptotic function in respons to anticancer drug in prostate cancer. Cancer Res 66(6):3114–3119
Ashery U, Yizhar O, Rotblat B et al (2006) Spatiotemporal organization of ras signaling: rasosomes and the galectin switch. Cell Mol Neurobiol 26:471–495
Shimura T, Takenaka Y, Tsutsumi S et al (2004) Galectin-3, a novel binding partner of beta-catenin. Cancer Res 64:6363–6367
Shimura T, Takenaka Y, Fukumori T et al (2005) Implication of galectin-3 in Wnt signaling. Cancer Res 65:3535–3537
Paron I, Scaloni A, Pines A et al (2003) Nuclear localization of Galectin-3 in transformed thyroid cells: a role in transcriptional regulation. Biochem Biophys Res Commun 302:545–553
Nangia-Makker P, Balan V, Raz A (2008) Regulation of tumor progression by extracellular galectin-3. Cancer Microenviron 1(1):43–51
Abdel-Aziz HO, Murai Y, Takasaki I et al (2008) Targeted disruption of the galectin-3 gene results in decreased susceptibility to NNK-induced lung tumorigenesis: An oligonucleotide microarray study. J Cancer Res Clin Oncol 134(7):777–788
Vereecken P, Zouaoui Boudjeltia K, Debray C et al (2005) High serum galectin-3 in advanced melanoma: preliminary results. Clin Exp Dermatol 31(1):105–109
Dittmar T, Heyder C, Gloria-Maercker E et al (2008) Adhesion molecules and chemokines: The navigation system for circulating tumor (stem) cells to metastasize in an organ-specific manner. Clin Exp Metastasis 25:11–32
Dennis JW, Laferté S, Waghorne C et al (1987) Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science 236:582–585
Lu Y, Pelling JC, Chaney WG (1994) Tumor cell surface b1–6 linked branched oligosaccharides and lung metastasis. Clin Exp Metast 12:47–54
Chakraborty AK, Pawelek J, Ikeda Y et al (2001) Fusion hybrids with macrophage and melanoma cells up-regulate N-acetylglucosaminyltransferase V, beta1-6 branching, and metastasis. Cell Growth Diff 12:623–630
Khaldoyanidi SK, Glinsky VV, Sikora L et al (2003) MDA-MB-435 human breast carcinoma cell homo- and heterotypic adhesion under flow conditions is mediated in part by Thomsen-Friedenreich antigen-galectin-3 interactions. J Biol Chem 278:4127–4134
Zhao Q, Barclay M, Hilkens J et al (2010) Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol Cancer 9:154
Ghiringhelli F, Menard C, Terme M et al (2005) CD4+CD25+ regulatory T cells inhibit natural killer cell function in a transforming growth factor-β-dependent manner. J Exp Med 202(8):1075–1085
Smyth MJ, Teng MWL, Swann J et al (2006) CD4+CD25+ regulatory T cells suppress NK cell-mediated immunotherapy of cancer. J Immunol 176(3):1582–1587
Passos ST, Silver JS, O’Hara ACO et al (2010) IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol 184:1776–1783
Smyth MJ, Hayakawa Y, Takeda K et al (2002) New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer 2(11):850–861
Muller AJ, Scherle PA (2006) Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer 6:742
Javia LR, Rosenberg SA (2003) CD4+CD25+ suppressor lymphocytes in the circulation of patients immunized against melanoma antigens. J Immunother 26:85–93
Marshall NA, Christie LE, Munro LR et al (2004) Immunosuppressive regulatory T cells are abudant in the reactive lymphocytes of Hodgkin lymphoma. Blood 103:1755–1762
Sutmuller RP, van Duivenvoorde LM, van Elsas A et al (2001) Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative patways for suppression of autoreactive cytotoxic T lymphocyte response. J Exp Med 194:823–832
Kryczek I, Wei S, Szeliga W et al (2009) Endogenous I-17 contributes to reduced tumor growth and metastasis. Blood 114(2):357–359
Zou W, Restifo NP (2010) Th17 cells in tumour immunity and immunotherapy. Nat Rev Immunol 10:248–256
Ishigami S, Natsugoe S, Tokuda K et al (2000) Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 88:577–583
Villegas FR, Coca S, Villarrubia VG et al (2002) Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 35:23–28
Whiteside TL, Herberman RB (1994) Role of human natural killer cells in health and disease. Clin Diag Lab Immunol 1:125–133
Hayakawa Y, Smyth MJ (2006) CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol 176:1517–1524
Ito M, Maruyama T, Saito N et al (2006) Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J Exp Med 203:289–295
Beyersdorf NB, Ding X, Karp K et al (2001) Expression of inhibitory “killer cell lectin-like receptor G1” identifies unique subpopulations of effector and memory CD8 T cells. Eur J Immunol 31:3443–3452
Andrews DM, Scalzo AA, Yokoyama WM et al (2003) Functional interactions between dendritic cells NK cells during viral infection. Nat Immunol 4:175–181
Robbins SH, Nguyen KB, Takahashi N et al (2002) Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J Immunol 168:2585–2589
Huntigton ND, Tabarias H, Fairfax K et al (2007) NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol 178:4764–4770
Acknowledgments
This work was supported by grants from the Ministry of Science and Technological Development (project number 175071 and 175069), Serbia and from the Croatian Ministry of Science (0062004 and 0062007), Croatia. We thank Dragana Markovic and Milan Milojevic for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radosavljevic, G., Jovanovic, I., Majstorovic, I. et al. Deletion of galectin-3 in the host attenuates metastasis of murine melanoma by modulating tumor adhesion and NK cell activity. Clin Exp Metastasis 28, 451–462 (2011). https://doi.org/10.1007/s10585-011-9383-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-011-9383-y