Abstract
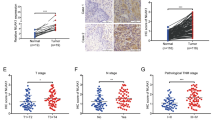
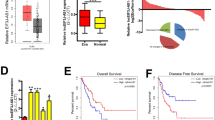
Esophageal cancer is characterized by rapid clinical progression and poor prognosis due to adjacent tissue invasion and distant organs metastasis at a very early stage. TM4SF3 (transmembrane 4 superfamily 3), a member of tetraspanin family, has been reported as a metastasis associated gene in many types of tumors. Herein, we described new properties of TM4SF3 in tumor metastasis, which suggested that this gene might be involved in esophageal carcinoma metastasis. Western blotting revealed that TM4SF3 was overexpressed in 57.1% (8/14) of esophageal carcinomas and esophageal carcinoma cell lines with high-invasive potential. Exogenous expression of TM4SF3 in two low-invasive esophageal carcinoma cell lines, KYSE150 and EC9706, significantly promoted cell migration and invasion. Upregulating TM4SF3 expression in EC9706 cells promoted xenograft tumor invading into surrounding tissues, enhanced lung metastasis, and shortened the lifespan of mice (median survival EC9706-TM4SF3 106.5 days versus EC9706-Vector 169.0 days, P < 0.0001) in a spontaneous metastasis model. Further studies demonstrated that ADAM12m was upregulated by TM4SF3 overexpression in vitro and in vivo. Abrogating up-expression of ADAM12m by siRNA significantly suppressed TM4SF3-mediated invasion. Together, these data from our studies indicated that overexpression of TM4SF3 in esophageal cancer conferred advantage to the invasion and metastasis of this destructive disease. Upregulated expression of ADAM12m by TM4SF3 might play a key role in TM4SF3-mediated invasion and metastasis. TM4SF3 and ADAM12m might be potential targets of esophageal carcinoma for anti-metastasis therapy.




Similar content being viewed by others
Abbreviations
- ADAM12:
-
A disintegrin and metalloproteinase
- EMT:
-
Epithelial-mesenchymal transition
- HE:
-
Hematoxylin and eosin
- MMP:
-
Metalloproteinase(s)
- RNAi:
-
RNA interference
- SiRNA:
-
Small interference RNA
- TM4SF3:
-
Transmembrane 4 superfamily 3
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Enzinger PC, Mayer RJ (2003) Esophageal cancer. N Engl J Med 349(23):2241–2252
Furihata T, Sakai T, Kawamata H et al (2001) A new in vivo model for studying invasion and metastasis of esophageal squamous cell carcinoma. Int J Oncol 19(5):903–907
Boucheix C, Rubinstein E (2001) Tetraspanins. Cell Mol Life Sci 58(9):1189–1205
Hemler ME (2005) Tetraspanin functions and associated microdomains. Nat Rev 6(10):801–811
Szala S, Kasai Y, Steplewski Z et al (1990) Molecular cloning of cDNA for the human tumor-associated antigen CO-029 and identification of related transmembrane antigens. Proc Natl Acad Sci USA 87(17):6833–6837
Kuhn S, Koch M, Nubel T et al (2007) A complex of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins promotes colorectal cancer progression. Mol Cancer Res 5(6):553–567
Kanetaka K, Sakamoto M, Yamamoto Y et al (2001) Overexpression of tetraspanin CO-029 in hepatocellular carcinoma. J Hepatol 35(5):637–642
Kanetaka K, Sakamoto M, Yamamoto Y et al (2003) Possible involvement of tetraspanin CO-029 in hematogenous intrahepatic metastasis of liver cancer cells. J Gastroenterol Hepatol 18(11):1309–1314
Zoller M (2006) Gastrointestinal tumors: metastasis and tetraspanins. Z Gastroenterol 44(7):573–586
Herlevsen M, Schmidt DS, Miyazaki K et al (2003) The association of the tetraspanin D6.1A with the alpha6beta4 integrin supports cell motility and liver metastasis formation. J Cell Sci 116(Pt 21):4373–4390
Gesierich S, Paret C, Hildebrand D et al (2005) Colocalization of the tetraspanins, CO-029 and CD151, with integrins in human pancreatic adenocarcinoma: impact on cell motility. Clin Cancer Res 11(8):2840–2852
Gesierich S, Berezovskiy I, Ryschich E et al (2006) Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res 66(14):7083–7094
Bick RL (1992) Coagulation abnormalities in malignancy: a review. Semin Thromb Hemost 18(4):353–372
Gschwind A, Hart S, Fischer OM et al (2003) TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. Embo J 22(10):2411–2421
Chandrasekar B, Bysani S, Mummidi S (2004) CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, I kappa B kinase, and nuclear factor-kappa B and induces cell-cell adhesion and aortic smooth muscle cell proliferation. J Biol Chem 279(5):3188–3196
Rhodes DR, Yu J, Shanker K et al (2004) ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia (New York, NY 6(1):1–6
Gu Z, Cui J, Brown S et al (2005) A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci 25(27):6401–6408
Mannello F, Sebastiani M (2003) Zymographic analyses and measurement of matrix metalloproteinase-2 and -9 in nipple aspirate fluids. Clin Chem 49(9):1546–1550
Yang Z, Kyriakides TR, Bornstein P (2000) Matricellular proteins as modulators of cell-matrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell 11(10):3353–3364
Lunter PC, van Kilsdonk JW, van Beek H et al (2005) Activated leukocyte cell adhesion molecule (ALCAM/CD166/MEMD), a novel actor in invasive growth, controls matrix metalloproteinase activity. Cancer Res 65(19):8801–8808
Agarwal R, D’Souza T, Morin PJ (2005) Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res 65(16):7378–7385
Huang J, Bridges LC, White JM (2005) Selective modulation of integrin-mediated cell migration by distinct ADAM family members. Mol Biol Cell 16(10):4982–4991
Luo ML, Shen XM, Zhang Y et al (2006) Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res 66(24):11690–11699
Shen XM, Wu YP, Feng YB et al (2007) Interaction of MT1-MMP and laminin-5gamma2 chain correlates with metastasis and invasiveness in human esophageal squamous cell carcinoma. Clin Exp Metastasis 24(7):541–550
Chu YW, Yang PC, Yang SC et al (1997) Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am J Respir Cell Mol Biol 17(3):353–360
Sato H, Takino T, Okada Y et al (1994) A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370(6484):61–65
Deryugina EI, Ratnikov B, Monosov E et al (2001) MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res 263(2):209–223
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3(5):362–374
Seals DF, Azucena EF Jr, Pass I et al (2005) The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell 7(2):155–165
Masson V, de la Ballina LR, Munaut C et al (2005) Contribution of host MMP-2 and MMP-9 to promote tumor vascularization and invasion of malignant keratinocytes. Faseb J 19(2):234–236
Liotta LA, Steeg PS, Stetler-Stevenson WG (1991) Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64(2):327–336
Roy R, Wewer UM, Zurakowski D et al (2004) ADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stage. J Biol Chem 279(49):51323–51330
Le Pabic H, Bonnier D, Wewer UM et al (2003) ADAM12 in human liver cancers: TGF-beta-regulated expression in stellate cells is associated with matrix remodeling. Hepatology 37(5):1056–1066
Carl-McGrath S, Lendeckel U, Ebert M et al (2005) The disintegrin-metalloproteinases ADAM9, ADAM12, and ADAM15 are upregulated in gastric cancer. Int J Oncol 26(1):17–24
Iba K, Albrechtsen R, Gilpin BJ et al (1999) Cysteine-rich domain of human ADAM 12 (meltrin alpha) supports tumor cell adhesion. Am J Pathol 154(5):1489–1501
Thodeti CK, Frohlich C, Nielsen CK et al (2005) ADAM12-mediated focal adhesion formation is differently regulated by beta1 and beta3 integrins. FEBS Lett 579(25):5589–5595
Acknowledgments
This work was supported by grants from the National key basic research program (NKBRP) (973 program) (No. 2002CB513106) and the National Natural Science Foundation (30570818 and 30600279).We thank Professor Mingrong Wang (Cancer Institute/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China) for the kind supply the EC9706 cell line and Dr. Shimada Y for the KYSE esophageal cancer cells (Kyoto University Graduate School of Medicine, Japan).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Case no. | Age (year) | Gender | Grade | TNM Stage |
|---|---|---|---|---|
1 | 48 | M | G3 | T3N0M0 |
2 | 58 | F | G3 | T2N0M0 |
3 | 51 | M | G2 | T4N1M0 |
4 | 52 | M | G2 | T3N0M1 |
5 | 63 | M | G2 | T3N1M0 |
6 | 55 | M | G3 | T3N1M0 |
7 | 61 | M | G1 | T2N0M0 |
8 | 62 | M | G3 | T2N0M0 |
9 | 66 | M | G1 | T3N1M) |
10 | 62 | F | G3 | T3N1M0 |
11 | 68 | M | G2 | T2N0M0 |
12 | 59 | M | G3 | T3N1M0 |
13 | 62 | M | G2 | T3N2M0 |
14 | 67 | M | G1 | T3N1M0 |
Rights and permissions
About this article
Cite this article
Zhou, Z., Ran, YL., Hu, H. et al. TM4SF3 promotes esophageal carcinoma metastasis via upregulating ADAM12m expression. Clin Exp Metastasis 25, 537–548 (2008). https://doi.org/10.1007/s10585-008-9168-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-008-9168-0