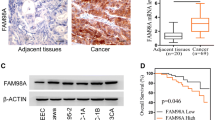
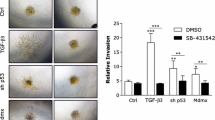
Abstract
Dominant negative (DN) mutations of tumor suppressor p53 (TP53) are clinically associated with cancer progression and metastasis of endometrial malignancy. To investigate the DN effect on tumor migration and invasion, we generated cells that stably co-expressed wild-type (wt) and R273H DN mutant TP53 (273H cells), and wt and R213Q recessive mutant TP53 (213Q cells), by transfection in endometrial cancer cells HHUA that expressed wt p53. R273H, but not R213Q, repressed wt p53-stimulated transcription of p21, Bax, and MDM2. 273H cells also showed markedly increased in vitro invasion and migration potentials, and displayed reduced Maspin, PAI-1, and KAI1 mRNA expressions as compared with 213Q and wt cells. The induction of wt p53 function by use of Adriamycin resulted in the inhibition of the invasion/migration capacity in association with the up-regulation of p53 target genes to a far greater degree in 213Q and wt cells than in 273H cells. R273H expression in p53-null cancer cell SK-OV-3 and Saos-2 did not significantly affect cell invasion and migration activities. Taken together, these results suggest that transdominance of R273H mutant over wt p53 rather than a gain-of-function promotes tumor metastasis by increasing invasion and migration in HHUA cells.








Similar content being viewed by others
References
Jemal A et al (2005) Cancer statistics. CA Cancer J Clin 55:10–30
Parazzini F, Lavecchia C, Bocciolone L, Franceschi S (1998) The epidemiology of endometrial cancer. Gynecol Oncol 41:1–16
Sakuragi N, Hirai A, Tada M, Yamada H, Yamamoto R, Fujimoto S, Moriuchi T (2001) Dominant-negative mutation of p53 tumor suppressor gene in endometrial carcinoma. Gynecol Oncol 83:485–490
Harris SL, Levine AJ (2005) The p53 pathway: positive and negative feedback loops. Oncogene 24:2899–2908
George SJ, Angelini GD, Capogrossi MC, Baker AH (2001) Wild-type p53 gene transfer inhibits neointima formation in human saphenous vein by modulation of smooth muscle cell migration and induction of apoptosis. Gene Ther 8:668–676
Guo F, Gao F, Wang L, Zheng Y (2003) p19Arf-p53 tumor suppressor pathway regulates cell motility by suppression of phosphoinositide 3-kinase and Rac1 GTPase activities. J Biol Chem 278:14414–14419
Kunz C, Pebler S, Otte J, von der Ahe D (1995) Differential regulation of plasminogen activated and inhibitor gene transcription by the tumor suppressor p53. Nucleic Acids Res 23:3710–3717
Zou Z et al (2000) p53 regulates the expression of the tumor suppressor gene Maspin. J Biol Chem 275:6051–6054
Marreiros A, Dudgeon K, Dao V, Grimm MO, Czolij R, Crossley M, Jackson P (2005) KAI1 promoter activity is dependent on p53, junB and AP2: evidence for a possible mechanism underlying loss of KAI1 expression in cancer cell. Oncogene 24:637–649
Willis A, Jung EJ, Wakefield T, Chen X (2004) Mutant p53 exerts a dominant negative effect by preventing wild-type p53 from binding to the promoter of its target genes. Oncogene 23:2330–2338
Milner J, Medcalf EA (1991) Cotranslation of activated mutant p53 with wild type drives the wild-type p53 protein into the mutant conformation. Cell 65:765–774
Sakuragi N et al (2005) Functional analysis of p53 gene and the prognostic impact of dominant-negative p53 mutation in endometrial cancer. Int J Cancer 116:514–519
Flaman JM et al (1995) A simple p53 functional assay for screening cell lines, blood, and tumors. Proc Natl Acad Sci USA 92:3963–3967
Takahashi M et al (2000) Distinct prognostic values of p53 mutations and loss of estrogen receptor and their cumulative effect in primary breast cancers. Int J Cancer 89:92–99
Hamada J et al (2001) Overexpression of homeobox gene HOXD3 induces coordinate expression of metastasis-related genes in human lung cancer cells. Int J Cancer 93:516–525
Fujita H, Okada F, Hamada J, Hosokawa M, Moriuchi T, Koya RC, Kuzumaki N (2001) Gelsolin functions as a metastasis suppressor in B16-BL6 mouse melanoma cells and requirement of the carboxyl-terminus for its effect. Int J Cancer 93:773–780
Cerda SR et al (2006) Protein kinase C delta inhibits Caco-2 cell proliferation by selective changes in cell cycle and cell death regulators. Oncogene 25:3123–3138
Flaman JM et al (1994) A rapid PCR fidelity assay. Nucl Acids Res 22:3259–3260
Smardova J, Pavlova S, Koukalova H (2002) Determination of optimal conditions for analysis of p53 status in leukemic cells using functional analysis of separated alleles in yeast. Pathol Oncol Res 8:245–251
Flaman JM et al (1996) The human tumour suppressor gene p53 is alternatively spliced in normal cells. Oncogene 12:813–818
Pocard M, Chevillard S, Villaudy J, Poupon MF, Dutrillaux B, Remvikos Y (1996) Different p53 mutations produce distinct effects on the ability of colon carcinoma cells to become blocked at the G1/S boundary after irradiation. Oncogene 15:875–882
Marutani M et al (1999) Dominant-negative mutations of the tumor suppressor p53 relating to early onset of glioblastoma multiforme. Cancer Res 59:4765–4769
Foster BA, Coffey HA, Morin MJ, Rastinejad F (1999) Pharmacological rescue of mutant p53 conformation and function. Science 286:2507–2510
Wang W, Takimoto R, Rastinejad F, El-Deiry WS (2003) Stabilization of p53 by CP-31398 inhibits ubiquitination without altering phosphorylation at serine 15 or 20 or MDM2 binding. Mol Cell Biol 23:2171–2181
Kim E, Deppert W (2004) Transcriptional activities of mutant p53: when mutations are more than a loss. J Cell Biochem 93:878–886
Blagosklonny MV (2000) p53 from complexity to simplicity: mutant p53 stabilization, gain-of-function, and dominant-negative effect. FASEB J 14:1901–1907
Saksela O, Rifkin DB (1988) Cell-associated plasminogen activation: regulation and physiological functions. Ann Rev Cell Biol 4:93–126
Whitley BR, Palmieri D, Twerdi CD, Church FC (2004) Expression of active plasminogen activator inhibitor-1 reduces cell migration and invasion in breast and gynecological cancer cells. Exp Cell Res 296:151–162
Amir S, Margaryan NV, Odero-March V, Khalkhali-Ellis Z, Hendrix MJ (2005) Maspin regulates hypoxia-mediated stimulation of uPA/uPAR complex in invasive breast cancer cells. Cancer Biol Ther 4:400–406
Odero-Marah VA et al (2003) Maspin regulates different signaling pathways for motility and adhesion in aggressive breast cancer cells. Cancer Biol Ther 2:67–72
Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, Barrett JC (1995) KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 268:884–886
Yang X, Wei LL, Tang C, Slack R, Mueller S, Lippman ME (2001) Overexpression of KAI1 suppresses in vitro invasiveness and in vivo metastasis in breast cancer cells. Cancer Res 61:5284–5288
Dittmer D et al (1993) Gain of function mutations in p53. Nat Genet 4:42–46
Blagosklonny MV, Giannakakou P, Romanova LY, Ryan KM, Vousden KH, Fojo T (2001) Inhibition of HIF-1- and wild-type p53-stimulated transcription by codon Arg175 p53 mutants with selective loss of functions. Carcinogenesis 22:861–867
Zhang L et al (2002) Combined anti-fetal liver kinase 1 monoclonal antibody and continuous low-dose doxorubicin inhibits angiogenesis and growth of human soft tissue sarcoma xenografts by induction of endothelial cell apoptosis. Cancer Res 62:2034–2042
Benbow U, Maitra R, Hamilton JW, Brinckerhoff CE (1999) Selective modulation of collagenase 1 gene expression by the chemotherapeutic agent doxorubicin. Clin Cancer Res 5:203–208
Repesh LA et al (1993) Adriamycin-induced inhibition of melanoma cell invasion is correlated with decreases in tumor cell motility and increases in focal contact formation. Clin Exp Metastasis 11:91–102
Nelson WG, Levy AA (1994) DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol 14:1815–1823
Hinds PW et al (1990) Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the “hot spot” mutant phenotypes. Cell Growth Differ 1:571–580
Duan W et al (2002) Lung-specific expression of human mutant p53-273H is associated with a high frequency of lung adenocarcinoma in transgenic mice. Oncogene 21:7831–7838
Bartek J, Iggo R, Gannon J, Lane DP (1990) Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene 5:893–899
Miller CW, Chumakov A, Said J, Chen DL, Also A, Koeffler HP (1993) Mutant p53 proteins have diverse intracellular abilities to oligomerize and activate transcription. Oncogene 8:1815–1824
Martinez LA et al (2002) p21 modulates threshold of apoptosis induced by DNA-damage and growth factor withdrawal in prostate cancer cells. Carcinogenesis 23:1289–1296
Acknowledgments
This study was partly supported by a grant for Clinical Cancer Research from the Ministry of Health, Labor, and Welfare. We thank Pfizer Inc., for providing the CP-31398 compound. The authors also thank Dr. Zhujie Xu for her kind and skillful help in Western blotting.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, P., Tada, M., Hamada, JI. et al. p53 dominant-negative mutant R273H promotes invasion and migration of human endometrial cancer HHUA cells. Clin Exp Metastasis 24, 471–483 (2007). https://doi.org/10.1007/s10585-007-9084-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-007-9084-8