Abstract
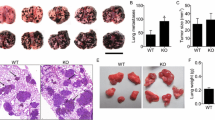
Previous studies conducted in our laboratory showed that the reproduction of spontaneous and experimental metastases was reduced in host animals deprived of essential fatty acids (EFA). In the present study, we have explored the possibility whether apoptosis, proliferation, and angiogenesis might be involved in the antimetastatic effect of EFA deficiency. To this aim, in pulmonary colonies developed from B16-F10 cells in EFA-deficient animals or in animals fed a 5% corn oil diet, we performed an immunohistochemical analysis of bcl-2/bax proteins, PCNA, and VEGF and von Willebrand Factor (vWF), typical markers of apoptosis, proliferation, and angiogenesis, respectively. Apoptosis was also evaluated by detecting DNA fragments in metastatic cells. We found that the reduction of pulmonary colonies grown in EFA-deficient animals was associated with a high expression of apoptotic activity as revealed by the presence of apoptotic nuclei and a high immunoreactivity for bax. Cell proliferation seemed not to be influenced by EFA deficiency in view of the observation that PCNA was highly expressed in pulmonary colonies of control as well as EFA-deficient animals. Pulmonary colonies developed in EFA- deficient animals showed a lower expression of VEGF and a decreased microvessel density, indicating that a reduced angiogenesis contributes to the antimetastatic effects of EFA deficiency. Our analysis of the results invokes the possibility that a relationship between angiogenesis and apoptosis may account for the diminution of the development of experimental metastases in the lungs of EFA-deficient animals.
Similar content being viewed by others
Abbreviations
- EFA:
-
essential fatty acids
- PUFA:
-
polyunsaturated fatty acids
- VWF:
-
von Willebrand Factor
References
Reddy BS (1994) Chemoprevention of colon cancer by dietary fatty acids. Cancer Metastasis Rev 13:285–302
Rose DP (1997) Dietary fatty acids and cancer. Am J Clin Nutr 66:998S–1003S
Willett WC (2001) Diet and breast cancer. J Intern Med 249:395–411
Carroll KK (1992) Dietary fat and breast cancer. Lipids 27:793–797
Ip C (1987) Fat and essential fatty acid in mammary carcinogenesis. Am J Clin Nutr 45:218–224
Kollmorgen GM, King MM, Kosanke SD, Do C (1983) Influence of dietary fat and indomethacin on the growth of transplantable mammary tumors in rats. Cancer Res 43:4714–4719
Reddy BS, Sugie S (1988) Effect of different levels of omega-3 and omega-6 fatty acids on azoxymethan induced colon carcinogenesis in F344 rats. Cancer Res 48:6642–6647
Roebuck BD, Longnecker DS, Baumgartner KJ et al (1985) Carcinogen-induced lesions in the rat pancreas: effects of varying levels of essential fatty acid. Cancer Res 45:5252–5256
Hubbard NE, Erikson KL (1987) Enhancement of metastasis from a transplantable mouse mammary tumor by dietary linoleic acid. Cancer Res 47:6171–6175
Katz EB, Boylan ES (1987) Stimulatory effect of high polyunsaturated fat diet on lung metastasis from the 13762 mammary adenocarcinoma in female retired breeder rats. J␣Natl Cancer Inst 79:351–358
Katz EB, Boylan ES (1989) Effect of the quality of dietary fat on tumor growth and metastasis from a rat mammary adenocarcinoma. Nutr Cancer 12:343–350
Rose DP, Hatala MA, Connolly JM et al (1993) Effect of diets containing different levels of linoleic acid on human breast cancer growth and lung metastasis in nude mice. Cancer Res 53:4686–4690
Rose DP, Connolly JM, Liu X-H (1994) Effects of linoleic acid on the growth and metastasis of two human breast cancer cell lines in nude mice and the invasive capacity of these cell lines in vitro. Cancer Res 54:6557–6562
Hubbard NE, Chapkin RS, Erickson KL (1988) Inhibition of growth and linoleate-enhanced metastasis of a transplantable mouse mammary tumor by indomethacin. Cancer Lett 43:111–120
Fulton AM (1988) The role of eicosanoids in tumor metastasis. Prostaglandins Leukot Essent Fatty Acids 34:229–237
Eynard AR, Quiroga P, Silva R (1989) Dietary fatty acid composition modulates the metastatic behavior of a murine mammary gland adenocarcinoma. Cell Biol Int Rep 13:813–814
Lala PK, Parhar RS, Singh P (1986) Indomethacin therapy abrogates the prostaglandin-mediated suppression of natural killer activity in tumor-bearing mice and prevents tumor metastasis. Cell Immunol 99:108–118
Calorini L, Ruggieri S (1992) Reduction of the metastatic potential of a RSV-transformed fibroblastic line (B77-AA6 cells) upon transplantation in essential fatty acid-deficient mice. Invasion Metastasis 12:233–240
Mannini A, Calorini L, Mugnai G et al (1998) Diminution of the development of experimental metastases produced by murine metastatic lines in essential fatty acid-deficient host mice. Clin Exp Metastasis 16:407–414
Townson JL, Naumov GN, Chambers AF (2003) The role of apoptosis in tumor progression and metastasis. Curr Mol Med 3:631–642
Granville DJ, Carthy CM, Hunt DW, McManus BM (1998) Apoptosis: molecular aspects of cell death and disease. Lab Invest 78:893–913
Coomber BL, Yu JL, Fathers KE et al (2003) Angiogenesis and the role of epigenetics in metastasis. Clin Exp Metastasis 20:215–227
Zetter BR (1998) Angiogenesis and tumor metastasis. Annu Rev Med 49:407–424
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29:15–18
Dunham EW, Balasingam M, Privett OS, Nickell EC (1978) Effects of essential fatty acid deficiency on prostaglandin synthesis and fatty acid composition in rat renal medulla. Lipids 13:892–897
Mathias MM, Dupont J (1979) The relationship of dietary fats to prostaglandin biosynthesis. Lipids 14:247–252
Croft KD, Beilin LJ, Vandongen R, Mathews E (1984) Dietary modification of fatty acid and prostaglandin synthesis in the rat. Effect of variations in the level of dietary fat. Biochim Biophys Acta 795:196–207
Parnham MJ, Vincent JE, Zijlstra FJ, Bonta IL (1979) The use of essential fatty acid deficient rats to study pathophysiological roles of prostaglandins. Comparison of prostaglandin production with some parameters of deficiency. Lipids 14:407–412
Tang DG, Chen YQ, Honn KV (1996) Arachidonate lipoxygenases as essential regulators of cell survival and apoptosis. Proc Natl Acad Sci USA 93:5241–5246
Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A (2004) Dietary long-chain n−3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr 79:935–945
Ghosh J, Myers CE (1998) Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci U S A 95:13182–13187
Rose DP, Connolly JM (2000) Regulation of tumor angiogenesis by dietary fatty acids and eicosanoids. Nutr Cancer 37:119–127
Form DM, Auerbach R (1983) PGE2 and angiogenesis. Proc Soc Exp Biol Med 172:214–218
Nie D, Tang K, Diglio C, Honn KV (2000) Eicosanoid regulation of angiogenesis: role of endothelial arachidonate 12-lipoxygenase. Blood 95:2304–2311
Collins MK, Lopez Rivas A (1993) The control of apoptosis in mammalian cells. Trends Biochem Sci 18:307–309
Rydén L, Linderholm B, Nielsen NH et al (2003) Tumor specific VEGF-A and VEGFR2/KDR protein are co- expressed in breast cancer. Breast Cancer Res Treat 82:147–154
Holley RW, Baldwin JH, Kiernan JA (1974) Control of growth of a tumor cell by linoleic acid. Proc Natl Acad Sci U S A 71:3976–3978
Korystov YuN, Shaposhnikova VV, Levitman MKh et al (1998) The effect of inhibitors of arachidonic acid metabolism on proliferation and death of tumor cells. FEBS Lett 431:224–226
Holman RT (1968) Essential fatty acid deficiency. In: Holman RT (eds). Progress in the chemistry on fats and other lipids. Pergamon Press, Oxford, pp 275–348
Fidler IJ (1973) Selection of successive tumour lines for metastasis. Nat New Biol 242:148–149
Chen TR (1977) In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res 104(2):255–262
Chan WY, Cheung KK, Schorge JO et al (2000) Bcl-2 and p53 protein expression, apoptosis, and p53 mutation in human epithelial ovarian cancers. Am J Pathol 156(2):409–417
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Eling TE, Glasgow WC (1990) Transduction of the epidermal growth factor mitogenic signal in BALB/c 3T3 fibroblasts involves linoleic acid metabolism. Adv Prostaglandin Thromboxane Leukot Res 21B:843–846
Holman RT (1998) The slow discovery of the importance of omega 3 essential fatty acids in human health. J Nutr 128:427S–433S
Heird WC, Lapillonne A (2005) The role of essential fatty acids in development. Annu Rev Nutr 25:549–571
Kasayama S, Koga M, Kouhara H et al (1994) Unsaturated fatty acids are required for continuous proliferation of transformed androgen-dependent cells by fibroblast growth factor family proteins. Cancer Res 54:6441–6445
Kidwell WR, Monaco ME, Wicha MS, Smith GS (1978) Unsaturated fatty acid requirements for growth and survival of a rat mammary tumor cell line. Cancer Res 38:4091–4100
Rose DP, Connolly JM (1989) Stimulation of growth of human breast cancer cell lines in culture by linoleic acid. Biochem Biophys Res Commun 164:277–283
Tombaccini D, Fallani A, Mugnai G, Ruggieri S (1981) Lipid composition of Balb/c3T3, SV3T3, and Concanavalin A-selected revertant cells grown in media containing lipid-depleted serum. J Lipid Res 22:590–597
Bailey JM, Dunbar LM (1971) Lipid metabolism in cultured cells: growth of tumor cells deficient in essential fatty acids. Cancer Res 31:91–97
Balakrishnan A, Cramer S, Bandyopadhyay GK et al (1989) Differential proliferative response to linoleate in cultures of epithelial cells from normal human breast and fibroadenomas. Cancer Res 49:857–862
Tang DG, Guan K-L, Li L et al (1997) Suppression of W256 carcinoma cell apoptosis by arachidonic acid and other polynsaturated fatty acids. Int J Cancer 72:1078–1087
Sun Y, Tang XM, Half E et al (2002) Cyclooxygenase-2 overexpression reduces apoptotic susceptibility by inhibiting the cytochrome c-dependent apoptotic pathway in human colon cancer cells. Cancer Res 62:6323–6328
Jantke J, Ladehoff M, Kurzel F et al (2004) Inhibition of the arachidonic acid metabolism blocks endothelial cell migration and induces apoptosis. Acta Neurochir (Wien) 146:483–494
Mukutmoni M, Hubbard NE, Erickson KL (2001) Prostaglandin E(2) modulation of vascular endothelial growth factor production in murine macrophages. Prostaglandins Leukot Essent Fatty Acids 65:123–131
Dubois RN (2000) Review article: cyclooxygenase–a target for colon cancer prevention. Aliment Pharmacol Ther 14:64–67
Williams CS, Mann M, DuBois RN (1999) The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18:7908–7916
Liu CH, Chang SH, Narko K et al (2001) Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem 276:18563–18569
Duff M, Stapleton PP, Mestre JR, et al (2003) Cyclooxygenase-2 inhibition improves macrophage function in melanoma and increases the antineoplastic activity of interferon gamma. Ann Surg Oncol 10:305–313
Lu X, Xie W, Reed D et al (1995) Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci USA 92:7961–7965
Dubois RN, Abramson SB, Crofford L et al (1998) Cyclooxygenase in biology and disease. FASEB J 12:1063–1073
Perrone G, Santini D, Verzi A, Vincenzi B, Borzomati D, Vecchio F, Coppola R, Antinori A, Magistrelli P, Tonini G, Rabitti C (2006) COX-2 expression in ampullary carcinoma: correlation with angiogenesis process and clinicopathological variables. J Clin Pathol 59:492–496
Acknowledgements
This work was supported by grants from MURST (60%) and ECRF (Italy).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mannini, A., Calzolari, A., Calorini, L. et al. The inhibition of lung colonization of B16-F10 melanoma cells in EFA-deficient animals is related to enhanced apoptosis and reduced angiogenesis. Clin Exp Metastasis 23, 159–165 (2006). https://doi.org/10.1007/s10585-006-9022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-006-9022-1