Abstract
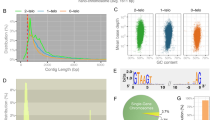
The dicyemid mesozoans are obligate parasites that inhabit the cephalopod renal appendage. Dicyemids have a simple body, consisting of approximately 30 cells: one long cylindrical axial cell contains intracellular stem cells (called axoblast), from which embryos are derived, and is surrounded by some 30 peripheral somatic cells. Somatic cells divide at most eight times in their life span, and never divide after differentiation. During early somatic cell development, numerous unique DNA sequences are first amplified and then eliminated, in the form of extrachromosomal circular DNA, leading to genome reduction. In this study we demonstrate that the remaining sequences, single-copy genes and repetitive sequences, have very different fates. Single-copy genes, such as β-tubulin, are initially amplified, presumably via endoreduplication, but subsequently decrease in copy number through development, suggesting that the whole genome is initially amplified and then the amplified DNAs are simply diluted in successive cell divisions, with little DNA replication. In contrast, repetitive sequences are maintained even in terminally differentiated somatic cell nuclei. Considering the increasing intensity of in-situ hybridization, incorporation of BrdU, and a general correlation between nuclear content and cell size, those repetitive sequences must be selectively endoreplicated in the peripheral cell nucleus, concomitant with the increase of cell size. The biological significance of this mechanism is discussed as a unique dicyemid adaptation to parasitism.
Similar content being viewed by others
References
Aeby P, Spicher A, de Chastonay Y, Müller F, Tobler H (1986) Structure and genomic organization of proretrovirus-like elements partially eliminated from the somatic genome of Ascaris lumbricoides. EMBO J 5: 3353–3360.
Albertson DG, Nwaorgu OC, Sulston JE (1979) Chromatin diminution and a chromosomal mechanism of sexual differentiation in Strongyloides papillosus. Chromosoma 75: 75–87.
Ammermann D, Steinbruck G, von Berger L, Hennig W (1974) The development of the macronucleus in the ciliated protozoan Stylonychia mytilus. Chromosoma 45: 401–429.
Awata H, Noto T, Endoh H (2005) Differentiation of somatic mitochondria and the structural changes in mtDNA during development of the dicyemid Dicyema japonicum (Mesozoa). Mol Genet Genom 273: 441–449.
Beaton MJ, Cavalier-Smith T (1999) Eukaryotic non-coding DNA is functional: evidence from the differential scaling of cryptomonad genomes. Proc Biol Sci 266: 2053–2059.
Brownlie JC, O'Neill SL (2005) Wolbachia genomes: insights into an intracellular lifestyle. Curr Biol 15: R507–R509.
Cavalier-Smith T (1978) Nuclear volume control by nucleoskeletal DNA, selection for cell volume and cell growth rate, and the solution of the DNA C-value paradox. J Cell Sci 34: 247–278.
Cavalier-Smith T (2005) Economy, speed and size matter: evolutionary forces driving nuclear genome miniaturization and expansion. Ann Bot (Lond) 95: 147–175.
Cavalier-Smith T, Beaton MJ (1999) The skeletal function of non-genic nuclear DNA: new evidence from ancient cell chimaeras. Genetica 106: 3–13.
Cohen S, Agmon N, Yacobi K, Mislovati M, Segal D (2005) Evidence for rolling circle replication of tandem genes in Drosophila. Nucleic Acids Res 33: 4519–4526.
Edgar BA, Orr-Weaver TL (2001) Endoreplication cell cycles: more for less. Cell 105: 297–306.
Embley TM, van der Giezen M, Horner DS, Dyal PL, Foster P (2003) Mitochondria and hydrogenosomes are two forms of the same fundamental organelle. Phil Trans R Soc Lond B Biol Sci 358: 191–202.
Etter A, Aboutanos M, Tobler H, Müller F (1991) Eliminated chromatin of Ascaris contains a gene that encodes a putative ribosomal protein. Proc Natl Acad Sci USA 88: 1593–1596.
Feagin JE (2000) Mitochondrial genome diversity in parasites. Int J Parasitol 30: 371–390.
Feagin JE, Gardner MJ, Williamson DH, Wilson RJ (1991) The putative mitochondrial genome of Plasmodium falciparum. J Protozool 38: 243–245.
Felder H, Herzceg A, de Chastonay Y et al (1994) Tas, a retrotransposon from the parasitic nematode Ascaris lumbricoides. Gene 149: 219–225.
Frank AC, Amiri H, Andersson SG (2002) Genome deterioration: loss of repeated sequences and accumulation of junk DNA. Genetica 115: 1–12.
Furuya H, Tsuneki K (2003) Biology of dicyemid mesozoans. Zool Sci 20: 519–532.
Furuya H, Tsuneki K, Koshida Y (1992) Development of the infusoriform embryo of Dicyema japonicum (Mesozoa: Dicyemidae). Biol Bull 183: 248–257.
Furuya H, Tsuneki K, Koshida Y (1994) The development of the vermiform embryos of two mesozoans, Dicyema acuticephalum and Dicyema japonicum. Zool Sci 11: 235–246.
Gray MW (1999) Evolution of organellar genomes. Curr Opin Genet Dev 9: 678–687.
Gray MW, Burger G, Lang BF (1999) Mitochondrial evolution. Science 283: 1476–1481.
Gregory TR (2001) Coincidence, coevolution, or causation? DNA content, cell size, and the C-value enigma. Biol Rev Camb Phil Soc 76: 65–101.
Gregory TR (2005) The C-value enigma in plants and animals: a review of parallels and an appeal for partnership. Ann Bot (Lond) 95: 133–146.
Gregory TR, Hebert PD, Kolasa J (2000) Evolutionary implications of the relationship between genome size and body size in flatworms and copepods. Heredity 84: 201–208.
Hartmann M (1906) Untersuchungen über den Generationswechsel der Dicyemiden. Mém Acad R Belg Ser II 1: 1–126.
Huang YJ, Stoffel R, Tobler H, Müller F (1996) A newly formed telomere in Ascaris suum does not exert a telomere position effect on a nearby gene. Mol Cell Biol 16: 130–134.
Hyman LH (1940) Phylum Mesozoa. In Shull F, ed. The Invertebrates 1. Protozoa through Ctenophora 1. New York: McGraw-Hill, pp. 233–247.
Katayama T, Wada H, Furuya H, Satoh N, Yamamoto M (1995) Phylogenetic position of the dicyemid mesozoa inferred from 18S rDNA sequences. Biol Bull 189: 81–90.
Kloc M, Zagrodzinska B (2001) Chromatin elimination–an oddity or a common mechanism in differentiation and development? Differentiation 68: 84–91.
Kobayashi M, Furuya H, Holland PW (1999) Dicyemids are higher animals. Nature 401: 762.
Kondorosi E, Roudier F, Gendreau E (2000) Plant cell-size control: growing by ploidy? Curr Opin Plant Biol 3: 488–492.
Kubota S, Takano J, Tsuneishi R et al (2001) Highly repetitive DNA families restricted to germ cells in a Japanese hagfish (Eptatretus burgeri): a hierarchical and mosaic structure in eliminated chromosomes. Genetica 111: 319–328.
Martin W (2005) The missing link between hydrogenosomes and mitochondria. Trends Microbiol 13: 457–459.
McConnaughey BH (1951) The life cycle of the dicyemid mesozoa. Univ Calif Publ Zool 55: 295–336.
Müller F, Tobler H (2000) Chromatin diminution in the parasitic nematodes Ascaris suum and Parascaris univalens. Int J Parasitol 30: 391–399.
Müller F, Bernard V, Tobler (1996) Chromatin diminution in nematodes. Bioessays 18: 133–138.
Niedermaier J, Moritz KB (2000) Organization and dynamics of satellite and telomere DNAs in Ascaris: implications for formation and programmed breakdown of compound chromosomes. Chromosoma 109: 439–452.
Noto T, Endoh H (2004) A ‘chimera’ theory on the origin of dicyemid mesozoans: evolution driven by frequent lateral gene transfer from host to parasite. Biosystems 73: 73–83.
Noto T, Yazaki K, Endoh H (2003) Developmentally regulated extrachromosomal circular DNA formation in the mesozoan Dicyema japonicum. Chromosoma 111: 359–368.
Nouvel H (1947) Les Dicyémides. 1re partie: systématique, générations vermiformes, infusorigène et sexualité. Arch Biol 58: 59–220.
Ohama T, Kumazaki T, Hori H, Osawa S (1984) Evolution of multicellular animals as deduced from 5S rRNA sequences: a possible early emergence of the Mesozoa. Nucleic Acids Res 12: 5101–5108.
Pawlowski J, Montoya-Burgos JI, Fahrni JF, Wuest J, Zaninetti L (1996) Origin of the Mesozoa inferred from 18S rRNA gene sequences. Mol Biol Evol 13: 1128–1132.
Prescott DM (1992) The unusual organization and processing of genomic DNA in hypotrichous ciliates. Trends Genet 8: 439–445.
Ridley RK (1968) Electron microscopic studies on dicyemid mesozoa. I. Vermiform stages. J Parasitol 54: 975–998.
Spano F, Crisanti A (2000) Cryptosporidium parvum: the many secrets of a small genome. Int J Parasitol 30: 553–565.
Spicher A, Etter A, Bernard V, Tobler H, Müller F (1994) Extremely stable transcripts may compensate for the elimination of the gene fert-1 from all Ascaris lumbricoides somatic cells. Dev Biol 164: 72–86.
Waters E, Hohn MJ, Ahel I et al (2003) The genome of Nanoarchaeum equitans: insights into early archaeal evolution and derived parasitism. Proc Natl Acad Sci USA 100: 12984–12988.
Wernegreen JJ (2002) Genome evolution in bacterial endosymbionts of insects. Nat Rev Genet 3: 850–861.
Wernegreen JJ (2005) For better or worse: genomic consequences of intracellular mutualism and parasitism. Curr Opin Genet Dev 15: 572–583.
Wu M, Sun LV, Vamathevan J et al. (2004) Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol 2: 327–341.
Zomorodipour A, Andersson SG (1999) Obligate intracellular parasites: Rickettsia prowazekii and Chlamydia trachomatis. FEBS Lett 452: 11–15.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Awata, H., Noto, T. & Endoh, H. Peculiar behavior of distinct chromosomal DNA elements during and after development in the dicyemid mesozoan Dicyema japonicum . Chromosome Res 14, 817–830 (2006). https://doi.org/10.1007/s10577-006-1084-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-006-1084-z