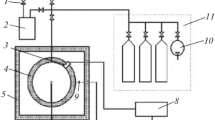
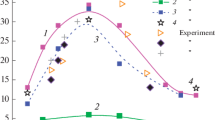
Abstract
Data obtained in the present work and available publications on combustion of cyclotetramethylene tetranitramine (HMX) at different initial temperatures are analyzed. The temperature sensitivity of the HMX burning rate is demonstrated to increase with increasing initial temperature at pressures of 0.1 to 10 MPa, which is typical for combustion of substances with the leading reaction in the condensed phase (c-phase model). Experimental values of the temperature sensitivity of the burning rate in the pressure interval between 0.1 and 1 MPa are higher than the values predicted by the c-phase model, but this fact indicates the transition of the combustion process to another regime rather than the combustion instability in this area. The flame structure of burning HMX with different additives is studied with the help of thin tungsten-rhenium thermocouples in the pressure range from 0.025 to 1 MPa. The gas-phase flame is found to ignite in an inductive mode, at least up to a pressure of 1 MPa. The surface temperature is obtained as a function of pressure on the basis of experimental data in a wide range of pressures: ln p = −14,092/T + 21.72 (p in atm). Two possible reasons for the oscillatory regime of HMX combustion observed at atmospheric pressure are proposed: the emergence of resonance phenomena during combustion of an inhomogeneous gas mixture in the tube and the lack of correspondence between the chemical reaction rate in the gas phase at the instant of the resonance and its energy capabilities, which do not allow a necessary HMX gasification rate to be ensured. A mechanism of HMX combustion is proposed, which offers an adequate description in a wide range of pressures up to 10 MPa. The mechanism is based on the leading role of HMX decomposition in the melt at the surface temperature.
Similar content being viewed by others
References
M. W. Beckstead and K. P. McCarty, “Calculated combustion characteristics of nitramine monopropellants, ” in: 13th JANNAF Combustion Meeting (1976), Vol. 1, pp. 57–68.
C. F. Price, T. L. Boggs, and R. L. Derr, “The steady-state combustion behavior of ammonium perchlorate and HMX, ” AIAA Paper No. 79-0164 (1979).
M. Ben-Reuven and L. Caveny, “Nitramine flame chemistry and deflagration interpreted in terms of flame model, ” AIAA Paper No. 79-1133 (1979).
N. Cohen, G. Lo, and J. Crowley, “Model and chemistry of HMX combustion, ” AIAA J., 23, No. 2, 276–282 (1985).
T. Mitani and F. A. Williams, “A model for the deflagration of nitramines, ” in: Proc. 21st Symp. (Int.) on Combustion, Combustion Institute (1986), pp. 1965–1974.
M. W. Beckstead, “Modeling AN, AP, HMX, and double base monopropellants, ” in: 26th JANNAF Combustion Meeting, CPIA Publ. No. 529, Vol. 4 (1989), pp. 255–268.
S. C. Li, F. A. Williams, and S. B. Margolis, “Effects of two-phase flow in a model for nitramine deflagration, ” Combust. Flame, 80, 329–349 (1990).
M. J. Ward, S. F. Son, and M. Q. Brewster, “Steady deflagration of HMX with simple kinetics: A gas phase chain reaction model, ” Combust. Flame, 114, Nos. 3–4, 556–568 (1998).
J. E. Davidson and M. W. Beckstead, “A three-phase model of HMX combustion, ” in: 26th Symp. (Int.) on Combustion, The Combustion Institute (1996), pp. 1989–1996.
K. Prasad, R. A. Yetter, and M. D. Smooke, “An eigenvalue method for computing the burning rates of RDX propellants, ” Combust. Sci. Technol., 124, 35–82 (1997).
T. L. Boggs, “The thermal behavior of cyclotrimethylenetrinitramine (RDX) and cyclotetramethylenetetranitramine (HMX), ” in: K. K. Kuo and M. Summerfield (eds.), Progress in Astronautics and Aeronautics, Vol. 90: Fundamentals of Solid-Propellant Combustion, Academic Press, New York (1984), pp. 121–175.
N. Kubota and S. Sakamoto, “Combustion mechanism of HMX, ” Propell., Explos., Pyrothechnics, 14, No. 1, 6–11 (1989).
A. Zenin, “HMX and RDX: Combustion mechanism and influence on modern double-base propellant combustion, ” J. Propuls. Power, 11, No. 4, 752–758 (1995).
A. A. Zenin, V. M. Puchkov, and S. V. Finjakov, “Characteristics of HMX combustion waves at various pressures and initial temperatures, ” Combust., Expl., Shock Waves, 34, No. 2, 170–176 (1998).
A. A. Zenin and S. V. Finjakov, “Characteristics of octogen and hexogen combustion: A comparison, ” in: Energetic Materials, Proc. 37th Int. Annu. Conf. of ICT, Karlsruhe, FRG (2006), pp. 154(1)–154(18).
N. E. Ermolin and V. E. Zarko, “Modeling of cyclic-nitramine combustion, ” Combust., Expl., Shock Waves, 34, No. 5, 485–501 (1998).
M. W. Beckstead, “Recent progress in modeling solid propellant combustion, ” Combust., Expl., Shock Waves, 42, No. 6, 623–641 (2006).
A. A. Zenin, “Comments to M. W. Beckstead’s paper “Recent progress in modeling solid propellant combustion, ” Combust., Expl., Shock Waves, 43, No. 2, 241–242 (2007).
M. W. Beckstead, “Condensed-phase control? Or gas-phase control?” Combust., Expl., Shock Waves, 43, No. 2, 243–245 (2007).
G. Lengelle, J. Duterque, and J. F. Trubert, “Physicochemical mechanisms of solid propellant combustion, ” in: V. Yang, T. B. Brill, and W. Z. Ren (eds.), Progress in Astronautics and Aeronautics, Vol. 185: Solid Propellant Chemistry, Combustion, and Motor Interior Ballistics, AIAA, Reston (2000), pp. 287–334.
A. F. Belyaev, “Combustion of explosives, ” Zh. Fiz. Khim., 12, No. 1, 93–99 (1938).
Ya. B. Zel’dovich, “Theory of combustion of propellants and explosives, ” Zh. Éksp. Teor. Fiz., 12, Nos. 11/12, 498–524 (1942).
G. V. Belov, “Thermodynamic analysis of combustion products at high temperature and pressure, ” Propell., Explos., Pyrotechnics, 23, 86–89 (1998).
A. I. Atwood, T. L. Boggs, P. O. Curran, and D. M. Hanson-Parr, “Burning rate of solid propellant ingredients. Part 1: Pressure and initial temperature effects, ” J. Propuls. Power, 15, No. 6, 740–742 (1999).
A. P. Glazkova, Data of the Combustion Laboratory of the Institute of Chemical Physics of the Russian Academy of Sciences (Database entitled “Flame” on combustion of explosives and powders), Mendeleev University of Chemical Technology of Russia (1990–1999).
O. P. Korobeinichev, L. V. Kuibida, and V. Zh. Madirbaev, “Investigation of the chemical structure of the HMX flame, ” Combust., Expl., Shock Waves, 20, No. 3, 282–285 (1984).
V. P. Sinditskii, V. Y. Egorshev, A. I. Levshenkov, and V. V. Serushkin, “Combustion of ammonium dinitramide. Part 1: Burning behavior, ” J. Propuls. Power, 22, No. 4, 769–776 (2006).
T. L. Boggs, C. F. Price, D. E. Zurn, et al., “Temperature sensitivity of deflagration rate of HMX, ” in: 13th JANNAF Combustion Meeting, CPIA Publ. No. 281, (1976), pp. 45–56.
T. P. Parr, T. L. Boggs, C. E. Price, and D. M. Hanson-Parr, “Measurements of temperature sensitivity of HMX burn rates, ” in: 19th JANNAF Combustion Meeting, CPIA Publ. No. 366, Vol. 1 (1982), pp. 281–288.
V. N. Simonenko, V. E. Zarko, and A. B. Kiskin, “Characterization of self-sustaining combustion of cyclic nitramines, ” in: Energetic Materials, Proc. 29th Int. Annu. Conf. of ICT, Karlsruhe (1998), pp. 169(1)–169(14).
A. D. Margolin and A. E. Fogel’zang, “Combustion of tetryl, ” Combust., Expl., Shock Waves, 2, No. 2, 6–11 (1966).
E. B. Washburn and M. W. Beckstead, “Modeling multiphase effects in the combustion of HMX and RDX, ” J. Propuls. Power, 22, No. 5, 938–946 (2006).
V. P. Sinditskii, V. Y. Egorshev, and M. V. Berezin, “Study on combustion of new energetic nitramines, ” in: Proc. 32th Int. Annu. Conf. of ICT, Karlsruhe (2001), Paper No. 59.
J. W. Taylor and R. J. Crookes, “Vapour pressure and enthalpy of sublimation of 1,3,5,7-Tetranitro-1,3,5,7-tetraazacyclooctane (HMX), ” J. Chem. Soc., Trans. I, 72, 723–729 (1976).
A. N. Ali, S. F. Son, B. W. Asay, et al., “High-irradiance laser ignition of explosives, ” Combust. Sci. Technol., 175, 1551–1571 (2003).
G. N. Kudva and T. A. Litzinger, “Comparison of laser- and pressure-driven thrust response of HMX, ” J. Propuls. Power, 18, No. 6, 1218–1226 (2002).
R. A. Yetter, F. L. Dryer, M. T. Allen, and J. L. Gatto, “Development of gas-phase reaction mechanism for nitramine combustion, ” J. Propuls. Power, 11, No. 4, 683–697 (1995).
P. F. Pokhil, O. I. Nefedova, and A. D. Margolin, “Anomalous dependence of the powder burning rate on initial temperature, ” Dokl. Akad. Nauk SSSR, 145, No. 4, 860–862 (1962).
G. N. Kudva, “A study of lazer and pressure-driven response measurements for propellants at low pressure, ” Ph. D. Thesis, Pennsylvania State University (2001).
J. M. Rosen and C. Dickenson, “Vapor pressures and heats of sublimation of some high melting organic explosives, ” J. Chem. Eng. Data, 14, 120–124 (1969).
R. B. Cundall, T. F. Palmer, and C. E. C. Wood, “Vapor pressures measurements of some organic explosives, ” J. Chem. Soc., Faraday Trans. I, 74, 1339–1345 (1978).
P. G. Hall, “Thermal decomposition and phase transitions in solid nitramines, ” Trans. Faraday Soc., 67, No. 3, 556–562 (1971).
Yu. A. Maksimov, “Boiling temperature and enthalpy of vaporization of liquid RDX and HMX, ” Zh. Fiz. Khim., 66, No. 2, 540–542 (1992).
A. P. Korobko, I. V. Levakova, S. V. Krasheninikov, et al., “Solubility of nitrocompounds in an active binder based on polyester urethane rubber and nitroglycerin, ” Vooruzhenie. Politika. Konversiya, No. 5, 69–74 (2002).
J.-S. Lee, C.-K. Hsu, and C.-L. Chang, “A study on the thermal decomposition behaviours on PETN, RDX, HNS and HMX, ” Thermochim. Acta, 392,393, 173–176 (2002).
G. Singh, S. P. Felix, and P. Soni, “Studies on energetic compounds. Part 28: Thermolysis of HMX and its plastic bonded explosives containing estane, ” Thermochim. Acta, 399, 153–165 (2003).
A. A. Paletsky, E. N. Volkov, and O. P. Korobeinichev, “HMX flame structure for combustion in air at a pressure of 1 atm, ” Combust., Expl., Shock Waves, 44, No. 6, 639–654 (2008).
T. P. Parr and D. M. Hanson-Parr, “Thermal properties measurements of solid rocket propellant oxidizers and binder materials as a function of temperature, ” J. Energ. Mater., 17, No. 1, 1–47 (1999).
Yu. Ya. Maksimov, “Thermal decomposition of RDX and HMX, ” in: Theory of Explosives, Papers of the Mendeleev University of Chemical Technology, Issue 53, Vysshaya Shkola, Moscow (1967), pp. 73–84.
J. C. Oxley, A. B. Kooh, R. Szekers, and W. Zhang, “Mechanism of nitramines thermolysis, ” J. Phys. Chem., 98, No. 28, 7004–7008 (1994).
A. I. B. Robertson, “The thermal decomposition of explosives. — II: Cyclotrimethylenetrinitramine and cyclotetramethylenetetranitramine, ” Trans. Faraday Soc., 45, 85–93 (1949).
C.E.H. Baun, in: W. E. Garner (ed.), Chemistry of the Solid State [Russian translation], Izd. Inostr. Lit., Moscow (1961), pp. 335–353.
G. Lengelle, “Thermal degradation kinetics and surface pyrolysis of vinyl polymers, ” AIAA J., 8, No. 11, 1989–1996 (1970).
A. G. Merzhanov and F. I. Dubovitskii, “Theory of steady combustion of powder, ” Dokl. Akad. Nauk SSSR, 129, No. 1, 153–156 (1959).
V. N. Simonenko, A. B. Kiskin, V. E. Zarko, and A. G. Svit, “Special features of nitramine combustion at atmospheric pressure, ” Combust., Expl., Shock Waves, 33, No. 6, 685–687 (1997).
C.-J. Tang, Y. Lee, and T. A. Litzinger, “Simultaneous temperature and species measurements during self-oscillating burning of HMX, ” J. Propuls. Power, 15, No. 2, 296–303 (1999).
B. V. Novozhilov, Unsteady Combustion of Solid Propellants [in Russian], Nauka, Moscow (1973).
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Fizika Goreniya i Vzryva, Vol. 45, No. 4, pp. 128–146, July–August, 2009.
Rights and permissions
About this article
Cite this article
Sinditskii, V.P., Egorshev, V.Y., Berezin, M.V. et al. Mechanism of HMX combustion in a wide range of pressures. Combust Explos Shock Waves 45, 461–477 (2009). https://doi.org/10.1007/s10573-009-0057-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10573-009-0057-x