Abstract
Hypothalamic–pituitary–adrenal (HPA) axis regulates stress response in the body and abnormal increase in oxidative stress contributes to the various disease pathogenesis. Although hypothalamic distribution of Apelin receptor (APLNR) has been studied, the potential regulatory role in hormone releasing function of hypothalamus in response to stress is not well elucidated yet. To determine whether APLNR is involved in the protection of the hypothalamus against oxidative stress, gonadotropin-releasing hormone (GnRH) cells were used as an in vitro model system. GT1-7 mouse hypothalamic neuronal cell line was subjected to H2O2 and hypoxia induced oxidative stress under various circumstances including APLNR overexpression, knockdown and knockout. Overexpression and activation of APLNR in GnRH producing neurons caused an increase in cell proliferation under oxidative stress. In addition, blockage of APLNR function by siRNA reduced GnRH release. Activation of APLNR initiated AKT kinase pathway as a proliferative response against hypoxic culture conditions and blocked apoptosis. Although expression and activation of APLNR have not been related to GnRH neuron differentiation during development, positive contribution of activated APLNR signaling to GnRH release in mouse embryonic stem cell derived GnRH neurons was observed in the present study. Sustained overexpression and complete deletion of APLNR in mouse embryonic stem cell derived GnRH neurons reduced GnRH release in vitro. The present findings suggest that expression and activation of APLNR in GnRH releasing GT1-7 neurons might induce a protective mechanism against oxidative stress induced cell death and APLNR signaling may play a role in GnRH neurons.









Similar content being viewed by others
Data Availability
Data are available upon request.
References
Aboutaleb N, Kalalianmoghaddam H, Eftekhari S, Shahbazi A, Abbaspour H, Khaksari M (2014) Apelin-13 inhibits apoptosis of cortical neurons following brain ischemic reperfusion injury in a transient model of focal cerebral ischemia. Int J Pept Res Ther 20:127–132
Atluri P et al (2007) Ischemic heart failure enhances endogenous myocardial apelin and apj receptor expression. Cell Mol Biol Lett 12:127–138
Bai B, Tang J, Liu H, Chen J, Li Y, Song W (2008) Apelin-13 induces Erk1/2 but not P38 mapk activation through coupling of the human apelin receptor to the Gi2 pathway. Acta Biochim Et Biophys Sin 40:311–318
Barron AM, Fuller SJ, Verdile G, Martins RN (2006) Reproductive hormones modulate oxidative stress in Alzheimer's disease. Antioxid Redox Signal 8:2047–2059
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci 87:1620–1624
Behan LA, Agha A (2007) Endocrine consequences of adult traumatic brain injury. Horm Res 68(Suppl 5):18–21. https://doi.org/10.1159/000110466
Bhagavath B et al (2006) Clinical and molecular characterization of a large sample of patients with hypogonadotropic hypogonadism. Fertil Steril 85:706–713
Bianco SD, Kaiser UB (2009) The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol 5:569
Bondanelli M et al (2010) Predictors of pituitary dysfunction in patients surviving ischemic stroke. J Clin Endocrinol Metab 95:4660–4668
Boyadjieva NI, Sarkar DK (2013) Microglia play a role in ethanol-induced oxidative stress and apoptosis in developing hypothalamic neurons. Alcohol Clin Exp Res 37:252–262
Brailoiu GC, Dun SL, Yang J, Ohsawa M, Chang JK, Dun NJ (2002) Apelin-immunoreactivity in the rat hypothalamus and pituitary. Neurosci Lett 327:193–197
Butterfield DA, Perluigi M, Sultana R (2006) Oxidative stress in Alzheimer's disease brain: new insights from redox proteomics. Eur J Pharmacol 545:39–50
Chan PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21:2–14
Chapman NA, Dupré DJ, Rainey JK (2014) The apelin receptor: physiology, pathology, cell signalling, and ligand modulation of a peptide-activated class A Gpcr. Biochem Cell Biol 92:431–440
Chen H et al (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14:1505–1517
Clarke S (2015) Acupuncture for ivf and assisted reproduction. J Aust Tradit Med Soc 21:57
Congress U (1990) Office of technology assessment, neurotoxicity identifying and controlling poisons of the nervous system. Ota-Ba-436. Us Government Printing Office, Washington
Cox CM, Dagostino SL, Miller MK, Heimark RL, Krieg PA (2006) Apelin, the ligand for the endothelial g-protein-coupled receptor, apj, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol 296:177–189
Darby E, Anawalt BD (2005) Male hypogonadism. Treat Endocrinol 4:293–309
Dauer W, Przedborski S (2003) Parkinson's disease: mechanisms and models. Neuron 39:889–909
De Mota N, Lenkei Z, Llorens-Cortès C (2000) Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology 72:400–407
Demirci S, Doğan A, Apdik H, Ec T, Gulluoglu S, Of B, Şahin F (2018) Cytoglobin inhibits migration through Pı3k/Akt/Mtor pathway in fibroblast cells. Mol Cell Biochem 437:133–142
Doğan A, Me Y, Şahin F, Av K, Palotás A, Aa R (2012) Differentiation of human stem cells is promoted by amphiphilic pluronic block copolymers. Int J Nanomed 7:4849
Doğan A, Demirci S, Apdik H, Ea A, Şahin F (2017) Dental pulp stem cells (Dpscs) increase prostate cancer cell proliferation and migration under in vitro conditions. Tissue Cell 49:711–718
Drougard A et al (2014) Hypothalamic apelin/reactive oxygen species signaling controls hepatic glucose metabolism in the onset of diabetes. Antioxid Redox Signal 20:557–573
Duan J et al (2019) Neuroprotective effect of apelin 13 on ischemic stroke by activating Ampk/Gsk-3β/Nrf2 signaling. J Neuroinflamm 16:24
Edinger AL et al (1998) An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol 72:7934–7940
Foussal C et al (2010) Activation of catalase by apelin prevents oxidative stress-linked cardiac hypertrophy. FEBS Lett 584:2363–2370
Goazigo AR-L, Morinville A, Burlet A, Llorens-Cortes C, Beaudet A (2004) Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology 145:4392–4400
Gu Z et al (2002) S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 297:1186–1190
Gyengesi E, Paxinos G, Andrews BZ (2012) Oxidative stress in the hypothalamus: the importance of calcium signaling and mitochondrial ros in body weight regulation. Curr Neuropharmacol 10:344–353
Hawrylycz MJ et al (2012) An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489:391–399
Hsu LJ et al (2000) Α-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol 157:401–410
Iacovino M et al (2011) Inducible cassette exchange: a rapid and efficient system enabling conditional gene expression in embryonic stem and primary cells. Stem Cells 29:1580–1588
Ishii M, Iadecola C (2015) Metabolic and non-cognitive manifestations of Alzheimer’s disease: the hypothalamus as both culprit and target of pathology. Cell Metab 22:761–776
Jiang Y et al (2018) Apelin-13 attenuates er stress-associated apoptosis induced by Mpp+ İn Sh-Sy5y cells. Int J Mol Med 42:1732–1740
Kelestimur H, Ozcan M, Kacar E, Alcin E, Yılmaz B, Ayar A (2012) Melatonin elicits protein kinase C-mediated calcium response in immortalized Gt1–7 Gnrh neurons. Brain Res 1435:24–28
Khaksari M, Aboutaleb N, Nasirinezhad F, Vakili A, Madjd Z (2012) Apelin-13 protects the brain against ischemic reperfusion injury and cerebral edema in a transient model of focal cerebral ischemia. J Mol Neurosci 48:201–208
Khan P et al. (2011) Functional Agonists Of The Apelin (Apj) receptor. In: Probe reports from the Nih molecular libraries program. National Center For Biotechnology Information (Us), Bethesda
Kobayashi H, Matsuda M, Fukuhara A, Komuro R, Shimomura I (2009) Dysregulated glutathione metabolism links to impaired insulin action in adipocytes american. J Physiol Endocrinol Metab 296:E1326–E1334
Kong H-L, Li Z-Q, Zhao S-M, Yuan L, Miao Z-L, Liu Y, Guan R-M (2015) Apelin-Apj effects of ginsenoside-Rb1 depending on hypoxia-induced factor 1α İn hypoxia neonatal cardiomyocytes. Chin J Integr Med 21:139–146
Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA (2012) National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 9:e1001356
Masri B, Van Den Berghe L, Sorli C, Knibiehler B, Audigier Y (2009) Apelin signalisation and vascular physiopathology. J Soc Biol 203:171
Meczekalski B, Katulski K, Czyzyk A, Podfigurna-Stopa A, Maciejewska-Jeske M (2014) Functional hypothalamic amenorrhea and its influence on women’s health. J Endocrinol Investig 37:1049–1056
Medhurst AD et al (2003) Pharmacological and immunohistochemical characterization of the Apj receptor and its endogenous ligand apelin. J Neurochem 84:1162–1172
Mesmin C, Fenaille F, Fo B, Tabet J-C, Ezan E (2011) Identification and characterization of apelin peptides in bovine colostrum and milk by liquid chromatography-mass spectrometry. J Proteome Res 10:5222–5231
Nass R, Ws E (2019) Physiologic and pathophysiologic alterations of the neuroendocrine components of the reproductive axis. In: Jerome FS, Robert LB (eds) Yen and Jaffe's reproductive endocrinology. Elsevier, Amsterdam, pp 473–519
Newson M, Pope G, Roberts E, Lolait S, O’carroll A (2013) Stress-dependent and gender-specific neuroregulatory roles of the apelin receptor in the hypothalamic–pituitary–adrenal axis response to acute stress. J Endocrinol 216:99
Nıckenıg G et al (2002) Redox-sensitive vascular smooth muscle cell proliferation is mediated By Gklf And Id3 in vitro and in vivo. FASEB J 16:1077–1086
Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, Filip M (2016) Oxidative stress in neurodegenerative diseases. Mol Neurobiol 53:4094–4125. https://doi.org/10.1007/S12035-015-9337-5
O’dowd BF et al (1993) A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 136:355–360
Odonnell LA, Agrawal A, Sabnekar P, Dichter MA, Lynch DR, Kolson DL (2007) Apelin, an endogenous neuronal peptide, protects hippocampal neurons against excitotoxic injury. J Neurochem 102:1905–1917
Ozcan M, Alcin E, Ayar A, Yılmaz B, Sandal S, Kelestimur H (2011) Kisspeptin-10 elicits triphasic cytosolic calcium responses in immortalized Gt1-7 Gnrh neurones. Neurosci Lett 492:55–58
Pédelacq J-D, Cabantous S, Tran T, Terwilliger TC, Waldo GS (2006) Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol 24:79–88
Poliandri A et al (2017) Generation of kisspeptin-responsive Gnrh neurons from human pluripotent stem cells. Mol Cell Endocrinol 447:12–22
Reaux-Le Goazigo A, Alvear-Perez R, Zizzari P, Epelbaum J, Bluet-Pajot M-T, Llorens-Cortes C (2007) Cellular localization of apelin and its receptor in the anterior pituitary: evidence for a direct stimulatory action of apelin on acth release. Am J Physiol Endocrinol Metab 292:E7–E15
Reaux A, Gallatz K, Palkovits M, Llorens-Cortes C (2002) Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience 113:653–662
Sakuma T, Nishikawa A, Kume S, Chayama K, Yamamoto T (2014) Multiplex genome engineering in human cells using All-İn-One Crıspr/Cas9 vector system. Sci Rep 4:1–6
Sandal S et al (2015) The effects of intracerebroventricular infusion of Apelin-13 on reproductive function in male rats. Neurosci Lett 602:133–138
Schally AV et al (1971a) Isolation and properties of the Fsh and Lh-releasing hormone. Biochem Biophys Res Commun 43:393–399
Schally AV et al (1971b) Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science 173:1036–1038
Shin K, Kenward C, Jk R (2011) Apelinergic system structure and function. Compr Physiol 8:407–450
Soleimani SMA, Ekhtiari H, Cadet JL (2016) Drug-induced neurotoxicity in addiction medicine: from prevention to harm reduction. In: Hamed E, Martin P (eds) Progress in brain research, vol 223. Elsevier, Amsterdam, pp 19–41
Tatemoto K et al (1998) Isolation and characterization of a novel endogenous peptide ligand for the human Apj receptor. Biochem Biophys Res Commun 251:471–476
Than A, Zhang X, Leow MK-S, Poh CL, Chong SK, Chen P (2014) Apelin attenuates oxidative stress in human adipocytes. J Biol Chem 289:3763–3774
Thannickal TC, Lai Y-Y, Siegel JM (2008) Hypocretin (orexin) and melanin concentrating hormone loss and the symptoms of Parkinson's disease. Brain 131:E87
Tsutsumi R, Webster NJ (2009) Gnrh pulsatility, the pituitary response and reproductive dysfunction. Endocr J 56:729–737
Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, Slukvin II (2010) A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 7:718–729
Wu D, Yotnda P (2011) Induction and testing of hypoxia in cell culture Jove. J Visual Exp 54:E2899
Yalvaç ME et al (2011) Differentiation and neuro-protective properties of immortalized human tooth germ stem cells. Neurochem Res 36:2227
Yang L et al (2014a) Erk1/2 mediates lung adenocarcinoma cell proliferation and autophagy induced by apelin-13. Acta Biochim Biophys Sin 46:100–111
Yang Y, Zhang X, Cui H, Zhang C, Zhu C, Li L (2014b) Apelin-13 Protects The Brain Against Ischemia/Reperfusion Injury Through Activating Pı3k/Akt And Erk1/2 signaling pathways. Neurosci Lett 568:44–49
Yang N, Li T, Cheng J, Tuo Q, Shen J (2019) Role of Apelin/Apj system in hypothalamic-pituitary axis. Clin Chim Acta 499:149
Zeng X-XI, Wilm TP, Sepich DS, Solnica-Krezel L (2007) Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev Cell 12:391–402
Zhen EY, Higgs RE, Gutierrez JA (2013) Pyroglutamyl apelin-13 identified as the major apelin isoform in human plasma. Anal Biochem 442:1–9
Acknowledgements
This study was supported by Yeditepe University. We would like to thank to Professor Pamela Mellon for donation of GT1-7 cell line to Bayram Yilmaz lab. We are grateful to Dr. Michael Kyba for sharing A2Lox.cre mouse embryonic stem (ES) cells. We would like to thank to Dr. Terry P. Yamaguchi for processing A2Lox.cre cell line propagation and transfer, respectively.
Funding
This study was supported by Yeditepe University.
Author information
Authors and Affiliations
Contributions
HBŞ and TBH conducted most of the cell culture experiments and oxidative stress modeling. SŞ performed siRNA experiments. BK completed all light and confocal microscopy experiments. DS and SS conducted knockout and overexpression experiments, respectively. MÖ did all flow cytometry experiments and data analysis. BY provided GT1-7 cells and conducted GT1-7 hypoxia experiments. FŞ and AD completed mouse embryonic stem cell differentiation and characterization experiments. The first draft of the manuscript was written by HBŞ and AD and all authors commented on previous versions of the manuscript. BY and FŞ edited the manuscript before submission. All authors read and approved the final manuscript. AD is corresponding author and designed all experiments and conducted in vitro assays together with team.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10571_2020_968_MOESM1_ESM.tif
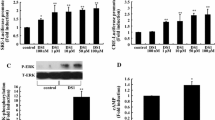
Supplementary Fig. 1. Total and phosphorylated Akt protein levels of GT1-7 cells under normoxia and oxidative stress conditions in vitro. The western blot band intensities of (A) Aplnr activation, (B) Aplnr knockdown, (C) Aplnr knockout, (D) Aplnr overexpression. Notes: Apelin-13: 1µM, ML233: 0.1µM, Protein amounts was normalized to β-actin, *P<0.05, **P<0.005, ***P<0.001, Akt: Protein kinase B, p-Akt: Phosphorylated Protein kinase B. (TIF 366 kb)
Rights and permissions
About this article
Cite this article
Şişli, H.B., Hayal, T.B., Şenkal, S. et al. Apelin Receptor Signaling Protects GT1-7 GnRH Neurons Against Oxidative Stress In Vitro. Cell Mol Neurobiol 42, 753–775 (2022). https://doi.org/10.1007/s10571-020-00968-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00968-2