Abstract
Chronic morphine-induced antinociceptive tolerance is a major unresolved issue in clinical practices, which is associated with microglia activation in the spinal cord. E3 ubiquitin ligase Pellino1 (Peli1) is known to be an important microglia-specific regulator. However, it is unclear whether Peli1 is involved in morphine tolerance. Here, we found that Peli1 levels in the spinal cord were significantly elevated in morphine tolerance mouse model. Notably, Peli1 was expressed in a great majority of microglia in the spinal dorsal horn, while downregulation of spinal Peli1 attenuated the development of morphine tolerance and associated hyperalgesia. Our biochemical data revealed that morphine tolerance-induced increase in Peli1 was accompanied by spinal microglia activation, activation of mitogen-activated protein kinase (MAPK) signaling, and production of proinflammatory cytokines. Peli1 additionally was found to promote K63-linked ubiquitination of tumor necrosis factor receptor-associated factor 6 (TRAF6) in the spinal cord after repeated morphine treatment. Furthermore, knocking down Peli1 in cultured BV2 microglial cells significantly attenuated inflammatory reactions in response to morphine challenge. Therefore, we conclude that the upregulation of Peli1 in the spinal cord plays a curial role in the development of morphine tolerance via Peli1-dependent mobilization of spinal microglia, activation of MAPK signaling, and production of proinflammatory cytokines. Modulation of Peli1 may be a potential strategy for the prevention of morphine tolerance.







Similar content being viewed by others
References
Bai L, Zhai C, Han K, Li Z, Qian J, Jing Y, Zhang W, Xu JT (2014) Toll-like receptor 4-mediated nuclear factor-kappaB activation in spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia in rats. Neurosci Bull 30(6):936–948. https://doi.org/10.1007/s12264-014-1483-7
Bekhit MH (2010) Opioid-induced hyperalgesia and tolerance. Am J Ther 17(5):498–510. https://doi.org/10.1097/MJT.0b013e3181ed83a0
Cai Y, Kong H, Pan YB, Jiang L, Pan XX, Hu L, Qian YN, Jiang CY, Liu WT (2016) Procyanidins alleviates morphine tolerance by inhibiting activation of NLRP3 inflammasome in microglia. J Neuroinflamm 13(1):53. https://doi.org/10.1186/s12974-016-0520-z
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63. https://doi.org/10.1016/0165-0270(94)90144-9
Chen Y, Sommer C (2009) The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence. Mol Neurobiol 40(2):101–107. https://doi.org/10.1007/s12035-009-8074-z
Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, Scherrer G (2017) Loss of mu opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med 23(2):164–173. https://doi.org/10.1038/nm.4262
Cuenda A, Rousseau S (2007) p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 1773(8):1358–1375. https://doi.org/10.1016/j.bbamcr.2007.03.010
Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX (2006) Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res 1069(1):235–243. https://doi.org/10.1016/j.brainres.2005.11.066
Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ (2008) A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun 22(1):114–123. https://doi.org/10.1016/j.bbi.2007.07.014
Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ (2000) Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103(2):351–361. https://doi.org/10.1016/s0092-8674(00)00126-4
Ferrini F, Trang T, Mattioli TA, Laffray S, Del'Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y (2013) Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat Neurosci 16(2):183–192. https://doi.org/10.1038/nn.3295
Grace PM, Hutchinson MR, Maier SF, Watkins LR (2014) Pathological pain and the neuroimmune interface. Nat Rev Immunol 14(4):217–231. https://doi.org/10.1038/nri3621
Grace PM, Maier SF, Watkins LR (2015) Opioid-induced central immune signaling: implications for opioid analgesia. Headache 55(4):475–489. https://doi.org/10.1111/head.12552
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32(1):77–88. https://doi.org/10.1016/0304-3959(88)90026-7
Huang XP, Peng JH, Pang JW, Tian XC, Li XS, Wu Y, Li Y, Jiang Y, Sun XC (2017) Peli1 contributions in microglial activation, neuroinflammatory responses and neurological deficits following experimental subarachnoid hemorrhage. Front Mol Neurosci 10:398. https://doi.org/10.3389/fnmol.2017.00398
Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR (2011) Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev 63(3):772–810. https://doi.org/10.1124/pr.110.004135
Imajo M, Tsuchiya Y, Nishida E (2006) Regulatory mechanisms and functions of MAP kinase signaling pathways. IUBMB Life 58(5–6):312–317. https://doi.org/10.1080/15216540600746393
Jiang Z, Johnson HJ, Nie H, Qin J, Bird TA, Li X (2003) Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J Biol Chem 278(13):10952–10956. https://doi.org/10.1074/jbc.M212112200
Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR (2004) A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci 24(33):7353–7365. https://doi.org/10.1523/JNEUROSCI.1850-04.2004
Kinsella S, Konig HG, Prehn JH (2016) Bid promotes K63-linked polyubiquitination of tumor necrosis factor receptor associated factor 6 (TRAF6) and sensitizes to mutant SOD1-induced proinflammatory signaling in microglia. eNeuro. https://doi.org/10.1523/ENEURO.0099-15.2016
Leduc-Pessah H, Weilinger NL, Fan CY, Burma NE, Thompson RJ, Trang T (2017) Site-specific regulation of P2X7 receptor function in microglia gates morphine analgesic tolerance. J Neurosci 37(42):10154–10172. https://doi.org/10.1523/JNEUROSCI.0852-17.2017
Liu DQ, Zhou YQ, Gao F (2019) Targeting cytokines for morphine tolerance: a narrative review. Curr Neuropharmacol 17(4):366–376. https://doi.org/10.2174/1570159X15666171128144441
Lu C, Hua F, Liu L, Ha T, Kalbfleisch J, Schweitzer J, Kelley J, Kao R, Williams D, Li C (2010) Scavenger receptor class-A has a central role in cerebral ischemia-reperfusion injury. J Cereb Blood Flow Metab 30(12):1972–1981. https://doi.org/10.1038/jcbfm.2010.59
Lu C, Liu L, Chen Y, Ha T, Kelley J, Schweitzer J, Kalbfleisch JH, Kao RL, Williams DL, Li C (2011) TLR2 ligand induces protection against cerebral ischemia/reperfusion injury via activation of phosphoinositide 3-kinase/Akt signaling. J Immunol 187(3):1458–1466. https://doi.org/10.4049/jimmunol.1003428
Lu C, Ha T, Wang X, Liu L, Zhang X, Kimbrough EO, Sha Z, Guan M, Schweitzer J, Kalbfleisch J, Williams D, Li C (2014a) The TLR9 ligand, CpG-ODN, induces protection against cerebral ischemia/reperfusion injury via activation of PI3K/Akt signaling. J Am Heart Assoc 3(2):e000629. https://doi.org/10.1161/JAHA.113.000629
Lu Y, Jiang BC, Cao DL, Zhang ZJ, Zhang X, Ji RR, Gao YJ (2014b) TRAF6 upregulation in spinal astrocytes maintains neuropathic pain by integrating TNF-alpha and IL-1beta signaling. Pain 155(12):2618–2629. https://doi.org/10.1016/j.pain.2014.09.027
Lueptow LM, Fakira AK, Bobeck EN (2018) The contribution of the descending pain modulatory pathway in opioid tolerance. Front Neurosci 12:886. https://doi.org/10.3389/fnins.2018.00886
Luo H, Winkelmann ER, Zhu S, Ru W, Mays E, Silvas JA, Vollmer LL, Gao J, Peng BH, Bopp NE, Cromer C, Shan C, Xie G, Li G, Tesh R, Popov VL, Shi PY, Sun SC, Wu P, Klein RS, Tang SJ, Zhang W, Aguilar PV, Wang T (2018) Peli1 facilitates virus replication and promotes neuroinflammation during West Nile virus infection. J Clin Invest 128(11):4980–4991. https://doi.org/10.1172/JCI99902
Marcus DJ, Zee M, Hughes A, Yuill MB, Hohmann AG, Mackie K, Guindon J, Morgan DJ (2015) Tolerance to the antinociceptive effects of chronic morphine requires c-Jun N-terminal kinase. Mol Pain 11:34. https://doi.org/10.1186/s12990-015-0031-4
Merighi S, Gessi S, Varani K, Fazzi D, Stefanelli A, Borea PA (2013) Morphine mediates a proinflammatory phenotype via mu-opioid receptor-PKCvarepsilon-Akt-ERK1/2 signaling pathway in activated microglial cells. Biochem Pharmacol 86(4):487–496. https://doi.org/10.1016/j.bcp.2013.05.027
Mika J (2008) Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep 60(3):297–307
Mika J, Wawrzczak-Bargiela A, Osikowicz M, Makuch W, Przewlocka B (2009) Attenuation of morphine tolerance by minocycline and pentoxifylline in naive and neuropathic mice. Brain Behav Immun 23(1):75–84. https://doi.org/10.1016/j.bbi.2008.07.005
Moynagh PN (2014) The roles of Pellino E3 ubiquitin ligases in immunity. Nat Rev Immunol 14(2):122–131. https://doi.org/10.1038/nri3599
Pan Y, Sun X, Jiang L, Hu L, Kong H, Han Y, Qian C, Song C, Qian Y, Liu W (2016) Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J Neuroinflamm 13(1):294. https://doi.org/10.1186/s12974-016-0754-9
Raghavendra V, Rutkowski MD, DeLeo JA (2002) The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci 22(22):9980–9989
Raghavendra V, Tanga FY, DeLeo JA (2004) Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology 29(2):327–334. https://doi.org/10.1038/sj.npp.1300315
Schauvliege R, Janssens S, Beyaert R (2007) Pellino proteins: novel players in TLR and IL-1R signalling. J Cell Mol Med 11(3):453–461. https://doi.org/10.1111/j.1582-4934.2007.00040.x
Song J, Zhu Y, Li J, Liu J, Gao Y, Ha T, Que L, Liu L, Zhu G, Chen Q, Xu Y, Li C, Li Y (2015) Pellino1-mediated TGF-beta1 synthesis contributes to mechanical stress induced cardiac fibroblast activation. J Mol Cell Cardiol 79:145–156. https://doi.org/10.1016/j.yjmcc.2014.11.006
Taves S, Berta T, Chen G, Ji RR (2013) Microglia and spinal cord synaptic plasticity in persistent pain. Neural Plast 2013:753656. https://doi.org/10.1155/2013/753656
Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412(6844):346–351. https://doi.org/10.1038/35085597
Watkins LR, Hutchinson MR, Johnston IN, Maier SF (2005) Glia: novel counter-regulators of opioid analgesia. Trends Neurosci 28(12):661–669. https://doi.org/10.1016/j.tins.2005.10.001
Wen YR, Tan PH, Cheng JK, Liu YC, Ji RR (2011) Microglia: a promising target for treating neuropathic and postoperative pain, and morphine tolerance. J Formos Med Assoc 110(8):487–494. https://doi.org/10.1016/S0929-6646(11)60074-0
Wu W, Hu Y, Li J, Zhu W, Ha T, Que L, Liu L, Zhu Q, Chen Q, Xu Y, Li C, Li Y (2014) Silencing of Pellino1 improves post-infarct cardiac dysfunction and attenuates left ventricular remodelling in mice. Cardiovasc Res 102(1):46–55. https://doi.org/10.1093/cvr/cvu007
Xia ZP, Sun L, Chen X, Pineda G, Jiang X, Adhikari A, Zeng W, Chen ZJ (2009) Direct activation of protein kinases by unanchored polyubiquitin chains. Nature 461(7260):114–119. https://doi.org/10.1038/nature08247
Xiao Y, Jin J, Chang M, Chang JH, Hu H, Zhou X, Brittain GC, Stansberg C, Torkildsen O, Wang X, Brink R, Cheng X, Sun SC (2013) Peli1 promotes microglia-mediated CNS inflammation by regulating Traf3 degradation. Nat Med 19(5):595–602. https://doi.org/10.1038/nm.3111
Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE (2008) TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol Cell 31(6):918–924. https://doi.org/10.1016/j.molcel.2008.09.002
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81600967 (Chen Lu), 81703493 (Tianya Liu)), the National Science Foundation for Young Scientists of Jiangsu Province (BK20150214 (Chen Lu), BK20170258 (Tianya Liu)), and Jiangsu Students for innovation and entrepreneurship training program (201610313065X (Chen Lu)).
Author information
Authors and Affiliations
Contributions
In this paper, LJW, CY, and YYX carried out cell experiments, animal surgery, and molecular biology studies. TYL and YZ performed the experimental data analysis. TYL and BW contributed reagents or analytic tools. CL and MA revised the manuscript. CL and JLC designed, supervised the study, and drafted the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All aspects of the animal care and experimental protocols were approved by Xuzhou Medical University Committee on Animal Care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10571_2020_797_MOESM1_ESM.docx
Fig. S1 The expression of GFP in the spinal dorsal horn. Representative immunofluorescence images showing the labeling of astrocytes (GFAP, red), microglial cells (Iba1, red), and neurons (NeuN, red) with GFP (green) in the spinal dorsal horn 7 days after the intrathecal injection. Scale bar represents 50 μm. n=3 per group. (DOCX 1334 kb)
10571_2020_797_MOESM2_ESM.docx
Fig. S2 Downregulation of spinal Peli1 does not reverse the established morphine tolerance. Morphine was administered subcutaneously twice daily for 11 days. Peli1 siRNA (siPeli1) or negative siRNA (siNeg) was intrathecally injected once daily from days 9 to 11 when morphine tolerance had fully developed. Each intrathecal injection is indicated by arrows. a Effectiveness of Peli1 siRNA or negative siRNA on expression of Peli1 were determined using western blot. Tissues were taken 4 hours after the last siRNA injection. n=4 per group. b MPE was determined using tail immersion test before and on day 8, 9, 10, 11 of morphine treatment. n=5 per group. c-d The paw withdrawal latency (c) and paw withdrawal threshold (d) were examined before and on day 11 of morphine treatment. n=5 per group. Results are expressed as the Mean ± SEM. *p<0.05 compared with indicated group. ns, statistically not significant. (DOCX 181 kb)
10571_2020_797_MOESM3_ESM.docx
Fig. S3 The effect of Peli1 on phosphorylated NF-B p65 levels after chronic morphine treatment in the spinal cord. Knockdown of spinal Peli1 did not attenuate morphine tolerance-induced increase in the phosphorylation of NF-B p65 (n=4 per group). Results are expressed as the Mean ± SEM. *p<0.05 compared with indicated group. ns, statistically not significant. (DOCX 108 kb)
10571_2020_797_MOESM4_ESM.docx
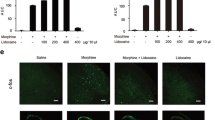
Fig. S4 Peli1 is involved in morphine-induced inflammatory reactions and microglia activation in BV2 cells. a BV2 cells were treated with or without 50 µM, 100 µM and 200 µM morphine for 24 h. Levels of Peli1 mRNA was determined using qPCR (n=4 per group). b-h BV2 cells were transfected with 75 µM Peli1 siRNA (siPeli1) or negative siRNA (siNeg) 24 h followed by the addition of morphine (200 µM) for 24 h. The expression of Peli1 mRNA (b, n=4 per group) and Peli1 protein (c, n=4 per group) were determined. Quantitative Western blot analysis showing the effect of Peli1 on P-NF-B p65 (d, n=4 per group) in BV2 microglial culture upon morphine treatment. e TNF-α (n=3-6 per group), IL-6 (n=4-6 per group), and IL-1β (n=4-6 per group) mRNA levels in BV2 cells were determined using qPCR. f After morphine stimulation, transfected BV2 cells were fixed and stained with DAPI (blue) and anti-Iba1 (red). Scale bar represents 20 m. n=3 per group. g qPCR analysis showing the inhibitory effect of Peli1 siRNA on Iba1 mRNA levels in BV2 cells after morphine treatment. n=4-5 per group. h Transfection of Peli1 siRNA inhibits the migration of BV2 cells into the scratched area. n=5 per group. Representative images were taken before and after scratch (4× magnification). Dashed line indicates the width of gap. Results are expressed as the Mean ± SEM. *p<0.05 compared with indicated group. ns, statistically not significant. (DOCX 1068 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Yin, C., Xu, X. et al. Pellino1 Contributes to Morphine Tolerance by Microglia Activation via MAPK Signaling in the Spinal Cord of Mice. Cell Mol Neurobiol 40, 1117–1131 (2020). https://doi.org/10.1007/s10571-020-00797-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00797-3