Abstract
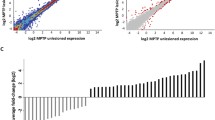
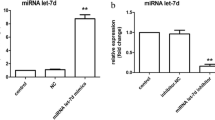
The glial cell line-derived neurotrophic factor (GDNF) potential as a therapeutic agent for the treatment of Parkinson’s Disease (PD) has been extensively explored. However, the mechanism of the GDNF neuroprotective effects is still unclear. In this study, the neuroprotective mechanism of the GDNF in the PD cellular models, which was obtained by the 6-hydroxydopamine (6-OHDA)-induced dopaminergic (DA) cell line MN9D damage was investigated by microarray. Interestingly, 54 constitutively increased or decreased genes were detected, 17 of which have not been reported previously. The expression of 5 up-regulated and 5 down-regulated genes which displayed the most obvious changes compared to the no GDNF treatment cells and was previously proven to be related to cell survival was validated by real-time PCR and western blot. Moreover, the up-regulated gene Ager and down-regulated gene Ccnl2 which were related to the PI-3K/Akt signaling pathway, but not researched in the neuron-cells, were investigated by overexpression and RNA interference. Overexpression of Ager or knockdown the expression of Ccnl2 decreased the damage to MN9D cells caused by 6-OHDA and reduced their apoptosis. All these results suggested that the protective effects of the GDNF on the 6-OHDA damaged MN9D cells could be understood by enhancing the expression of the apoptosis inhibiting genes and decreasing the expression of the apoptosis promoting genes. Thus, this study might provide a number of specific candidates and potential targets to investigate the protective mechanism of GDNF in DA neurons.







Similar content being viewed by others
References
Alves M, Calegari VC, Cunha DA et al (2005) Increased expression of advanced glycation end-products and their receptor, and activation of nuclear factor kappa-B in lacrimal glands of diabetic rats. Diabetologia 48:2675–2681
Bruchas MR, Macey TA, Lowe JD et al (2006) Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem 281:18081–18089
Cao JP, Wang HJ, Yu JK et al (2008) The involvement of NF-kappaB p65/p52 in the effects of GDNF on DA neurons in early PD rats. Brain Res Bull 76:505–511
Cao JP, Niu HY, Wang HJ et al (2013) NF-κB p65/p52 plays a role in GDNF up-regulating Bcl-2 and Bcl-w expression in 6-OHDA-induced apoptosis of MN9D cell. Int J Neurosci. doi:10.3109/00207454.2013.795149
Cavanaugh JE, Jaumotte JD, Lakoski JM et al (2006) Neuroprotective role of ERK1/2 and ERK5 in a dopaminergic cell line under basal conditions and in response tooxidative stress. J Neurosci Res 84:1367–1375
Choi HK, Won LA, Kontur PJ et al (1991) Immortalization of embryonic mesencephalic dopaminergic neurons by somatic cell fusion. Brain Res 552:67–76
Consales C, Volpicelli F, Greco D et al (2007) GDNF signaling in embryonic midbrain neurons in vitro. Brain Res 1159:28–39
Ding YM, Jaumotte JD, Signore AP et al (2004) Effects of 6-hydroxydopamine on primary cultures of substantia nigra: specific damage to dopamine neurons and the impact of glial cell line-derived neurotrophic factor. J Neurochem 89:776–787
Drinkut A, Tereshchenko Y, Schulz JB et al (2012) Efficient gene therapy for Parkinson’s disease using astrocytes as hosts for localized neurotrophic factor delivery. Mol Ther 20:534–543
Du Y, Li X, Yang D et al (2008) Multiple molecular pathways are involved in the neuroprotection of GDNF against proteasome inhibitor induced dopamine neuron degeneration in vivo. Exp Biol Med 233:881–890
Encinas M, Tansey MG, Tsui-Pierchala BA et al (2001) c-Src is required for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway. J Neurosci 21:1464–1472
Eslamboli A, Georgievska B, Ridley RM et al (2005) Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson’s disease. J Neurosci 25:769–777
Hetman M, Cavanaugh JE, Kimelman D et al (2000) Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci 20:2567–2574
Holtz WA, Turetzky JM, O’Malley KL (2005) Microarray expression profiling identifies early signaling transcripts associated with 6-OHDA-induced dopaminergic cell death. Antioxid Redox Signal 7:639–648
Imai Y, Soda M, Inoue H et al (2001) An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105:891–902
Ischiropoulos H, Beckman JS (2003) Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest 111:163–169
Kang R, Tang D, Livesey KM et al (2011) The Receptor for Advanced Glycation End-products (RAGE) protects pancreatic tumor cells against oxidative injury. Antioxid Redox Signal 15:2175–2184
Kim IS, Choi DK, Jung HJ (2011) Neuroprotective effects of vanillyl alcohol in Gastrodia elata Blume through suppression of oxidative stress and anti-apoptotic activity in toxin-induced dopaminergic MN9D cells. Molecules 16:5349–5361
Lansbury PT Jr, Brice A (2002) Genetics of Parkinson’s disease and biochemical studies of implicated gene products. Curr Opin Genet Dev 12:299–306
Lausson S, Cressent M (2011) Signal transduction pathways mediating the effect of adrenomedullin on osteoblast survival. J Cell Biochem 112:3807–3815
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1:120–129
Miyashita K, Itoh H, Arai H et al (2006) The neuroprotective and vasculo-neuro-regenerative roles of adrenomedullin in ischemic brain and its therapeutic potential. Endocrinology 147:1642–1653
Morrison TB, Weis JJ, Wittwer CT (1998) Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques 24:954–958
Park B, Oh CK, Choi WS et al (2011) Microarray expression profiling in 6-hydroxydopamine-induced dopaminergic neuronal cell death. J Neural Transm 118:1585–1598
Pascual A, Hidalgo-Figueroa M, Gómez-Díaz R et al (2011) GDNF and protection of adult central catecholaminergic neurons. J Mol Endocrinol 46:R83–R92
Perrinjaquet M, Vilar M, Ibáñez CF (2010) Protein-tyrosine phosphatase SHP2 contributes to GDNF neurotrophic activity through direct binding to phospho-Tyr687 in the RET receptor tyrosine kinase. J Biol Chem 285:31867–31875
Pieczenik SR, Neustadt J (2007) Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol 83:84–92
Ryu EJ, Harding HP, Angelastro JM et al (2002) Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci 22(24):10690–10698
Shimura H, Hattori N, Kubo S et al (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25:302–305
Su CM, Lu DY, Hsu CJ et al (2009) Glial cell-derived neurotrophic factor increases migration of human chondrosarcoma cells via ERK and NF-kappaB pathways. J Cell Physiol 220:499–507
Tanabe K, Matsushima-Nishiwaki R, Iida M et al (2012) Involvement of phosphatidylinositol 3-kinase/Akt on basic fibroblast growth factor-induced glial cell line-derived neurotrophic factor release from rat glioma cells. Brain Res 1463:21–29
Ugarte SD, Lin E, Klann E et al (2003) Effects of GDNF on 6-OHDA-induced death in a dopaminergic cell line: modulation by inhibitors of PI3 kinase and MEK. J Neurosci Res 73:105–112
Watanabe S, Ichimura T, Fujita N et al (2003) Methylated DNA-binding domain 1 and methylpurine-DNA glycosylase link transcriptional repression and DNA repair in chromatin. Proc Natl Acad Sci 100:12859–12864
Worby CA, Vega QC, Zhao Y et al (1996) Glial cell line-derived neurotrophic factor signals through the RET receptor and activates mitogen-activatedprotein kinase. J Biol Chem 271:23619–23622
Yang L, Li N, Wang C et al (2004) Cyclin L2, a novel RNA polymerase II-associated cyclin, is involved in pre-mRNA splicing and induces apoptosis of human hepatocellular carcinoma cells. J Biol Chem 279:11639–11648
Zhou A, Ou AC, Cho A et al (2008) Novel splicing factor RBM25 modulates Bcl-x pre-mRNA 5′ splice site selection. Mol Cell Biol 28:5924–5936
Zhuo L, Gong J, Yang R et al (2009) Inhibition of proliferation and differentiation and promotion of apoptosis by cyclin L2 in mouse embryonic carcinoma P19 cells. Biochem Biophys Res Commun 390:451–457
Acknowledgments
The present investigation was supported by the grants from the Natural Science Foundation of China (308707097), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Science Foundation of Xuzhou medical college (2012KJZ03).
Conflict of interest
The authors do not have any actual or potential conflicts of interest with other people or organizations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, F., Wang, M., Zhu, S. et al. The Potential Neuroprotection Mechanism of GDNF in the 6-OHDA-Induced Cellular Models of Parkinson’s Disease. Cell Mol Neurobiol 33, 907–919 (2013). https://doi.org/10.1007/s10571-013-9957-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-013-9957-0