Abstract
Women are twice as likely as men to suffer from stress-related psychiatric disorders, like unipolar depression and post-traumatic stress disorder. Although the underlying neural mechanisms are not well characterized, the pivotal role of stress in the onset and severity of these diseases has led to the idea that sex differences in stress responses account for this sex bias. Corticotropin-releasing factor (CRF) orchestrates stress responses by acting both as a neurohormone to initiate the hypothalamic–pituitary–adrenal (HPA) axis and as a neuromodulator in the brain. One target of CRF modulation is the locus coeruleus (LC)–norepinephrine system, which coordinates arousal components of the stress response. Hypersecretion of CRF and dysregulation of targets downstream from CRF, such as the HPA axis and LC–norepinephrine system, are characteristic features of many stress-related psychiatric diseases, suggesting a causal role for CRF and its targets in the development of these disorders. This review will describe sex differences in CRF and the LC–norepinephrine system that can increase stress sensitivity in females, making them vulnerable to stress-related disorders. Evidence for gonadal hormone regulation of hypothalamic CRF is discussed as an effect that can lead to increased HPA axis activity in females. Sex differences in the structure of LC neurons that create the potential for hyperarousal in response to emotional stimuli are described. Finally, sex differences at the molecular level of the CRF1 receptor that make the LC–norepinephrine system more reactive in females are reviewed. The implications of these sex differences for the treatment of stress-related psychiatric disorders also will be discussed.


Similar content being viewed by others
References
Abercrombie ED, Keller RW, Zigmond MJ (1988) Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience 27:897–904
Ahn S, Shenoy SK, Wei H, Lefkowitz RJ (2004) Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem 279(34):35518–35525. doi:10.1074/jbc
Amstadter AB, Nugent NR, Yang BZ, Miller A, Siburian R, Moorjani P, Haddad S, Basu A, Fagerness J, Saxe G, Smoller JW, Koenen KC (2011) Corticotrophin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis Markers 30(2–3):89–99. doi:10.3233/DMA-2011-0761
Arnold AP (2009) Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol 21(4):377–386. doi:10.1111/j.1365-2826.2009.01831.x
Arnold AP, Chen X (2009) What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 30(1):1–9. doi:10.1016/j.yfrne.2008.11.001
Aston-Jones G, Bloom FE (1981a) Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1:876–886
Aston-Jones G, Bloom FE (1981b) Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1:887–900
Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450
Aston-Jones G, Shipley MT, Grzanna R (1995) The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G (ed) The rat brain. Academic Press, San Diego, pp 183–213
Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT (1996) Role of the locus coeruleus in emotional activation. Prog Brain Res 107:380–402
Austin MC, Janosky JE, Murphy HA (2003) Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry 8(3):324–332
Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD Jr (1999) Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 156(4):585–588
Bangasser DA, Shors TJ (2008) The bed nucleus of the stria terminalis modulates learning after stress in masculinized but not cycling females. J Neurosci 28(25):6383–6387. doi:10.1523/JNEUROSCI.0831-08.2008
Bangasser DA, Santollo J, Shors TJ (2005) The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav Neurosci 119(6):1459–1466. doi:10.1037/0735-7044.119.6.1459
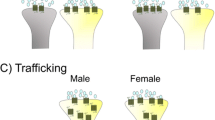
Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ (2010) Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15(9):877, 896–904. doi:10.1038/mp.2010.66
Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ (2011) Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav 103(3–4):342–351. doi:10.1016/j.physbeh.2011.02.037
Banki CM, Karmacsi L, Bissette G, Nemeroff CB (1992) Cerebrospinal fluid neuropeptides in mood disorder and dementia. J Affect Disord 25(1):39–45
Bao AM, Swaab DF (2007) Gender difference in age-related number of corticotropin-releasing hormone-expressing neurons in the human hypothalamic paraventricular nucleus and the role of sex hormones. Neuroendocrinology 85(1):27–36. doi:10.1159/000099832
Bao AM, Hestiantoro A, Van Someren EJ, Swaab DF, Zhou JN (2005) Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain 128(Pt 6):1301–1313. doi:10.1093/brain/awh448
Bao AM, Fischer DF, Wu YH, Hol EM, Balesar R, Unmehopa UA, Zhou JN, Swaab DF (2006) A direct androgenic involvement in the expression of human corticotropin-releasing hormone. Mol Psychiatry 11(6):567–576. doi:10.1038/sj.mp.4001800
Battaglia G, Webster EL, De Souza EB (1987) Characterization of corticotropin-releasing factor receptor-mediated adenylate cyclase activity in the rat central nervous system. Synapse 1(6):572–581. doi:10.1002/syn.890010610
Berridge CW, Foote SL (1991) Effects of locus coeruleus activation on electroencephalographic activity in the neocortex and hippocampus. J Neurosci 11:3135–3145
Berridge CW, Page ME, Valentino RJ, Foote SL (1993) Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience 55:381–383
Bingaman EW, Magnuson DJ, Gray TS, Handa RJ (1994) Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology 59(3):228–234
Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G (2003) Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology 28(7):1328–1335. doi:10.1038/sj.npp
Bohler HC Jr, Zoeller RT, King JC, Rubin BS, Weber R, Merriam GR (1990) Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Brain Res Mol Brain Res 8(3):259–262
Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS (1997) Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 154(5):624–629
Breslau N (2002) Gender differences in trauma and posttraumatic stress disorder. J Gend Specif Med 5(1):34–40
Breslau N (2009) The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse 10(3):198–210. doi:10.1177/1524838009334448
Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P (1998) Trauma and posttraumatic stress disorder in the community: the 1996 Detroit area survey of trauma. Arch Gen Psychiatry 55(7):626–632
Breslau N, Chilcoat HD, Kessler RC, Peterson EL, Lucia VC (1999) Vulnerability to assaultive violence: further specification of the sex difference in post-traumatic stress disorder. Psychol Med 29(4):813–821
Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lepine JP, Levinson D, Matschinger H, Mora ME, Browne MO, Posada-Villa J, Viana MC, Williams DR, Kessler RC (2011) Cross-national epidemiology of DSM-IV major depressive episode. BMC Med 9:90. doi:10.1186/1741-7015-9-90
Chen FM, Bilezikjian LM, Perrin MH, Rivier J, Vale W (1986) Corticotropin releasing factor receptor-mediated stimulation of adenylate cyclase activity in the rat brain. Brain Res 381(1):49–57
Cibelli G, Corsi P, Diana G, Vitiello F, Thiel G (2001) Corticotropin-releasing factor triggers neurite outgrowth of a catecholaminergic immortalized neuron via cAMP and MAP kinase signalling pathways. Eur J Neurosci 13(7):1339–1348
Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G (2004) Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J Neurosci 24(44):9914–9920. doi:10.1523/JNEUROSCI.2446-04.2004
Collins A, Frankenhaeuser M (1978) Stress responses in male and female engineering students. J Human Stress 4(2):43–48. doi:10.1080/0097840X.1978.9934986
Conti LH, Foote SL (1995) Effects of pretreatment with corticotropin-releasing factor (CRF) on the electrophysiological responsivity of the locus coeruleus to subsequent CRF challenge. Neuroscience 69:209–219
Conti LH, Foote SL (1996) Reciprocal cross-desensitization of locus coeruleus electrophysiological responsivity to corticotropin-releasing factor and stress. Brain Res 722:19–29
Conti LH, Youngblood KL, Printz MP, Foote SL (1997) Locus coeruleus electrophysiological activity and responsivity to corticotropin-releasing factor in inbred hypertensive and normotensive rats. Brain Res 774(1–2):27–34
Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS (1963) Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol 205(5):807–815
Curtis AL, Grigoriadis DE, Page ME, Rivier J, Valentino RJ (1994) Pharmacological comparison of two corticotropin-releasing factor antagonists: in vivo and in vitro studies. J Pharmacol Exp Ther 268(1):359–365
Curtis AL, Pavcovich LA, Grigoriadis DE, Valentino RJ (1995) Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience 65(2):541–550
Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ (1997) Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther 281(1):163–172
Curtis AL, Pavcovich LA, Valentino RJ (1999) Long-term regulation of locus ceruleus sensitivity to corticotropin-releasing factor by swim stress. J Pharmacol Exp Ther 289(3):1211–1219
Curtis AL, Bethea T, Valentino RJ (2006) Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31(3):544–554
Dalla C, Whetstone AS, Hodes GE, Shors TJ (2009) Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculinized females. Neurosci Lett 449(1):52–56. doi:10.1016/j.neulet.2008.10.051
Day HE, Curran EJ, Watson SJ Jr, Akil H (1999) Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol 413(1):113–128. doi:10.1002/(SICI)1096-9861(19991011)413:1<113:AID-CNE8>3.0.CO;2-B
De Bellis MD, Gold PW, Geracioti TD Jr, Listwak SJ, Kling MA (1993) Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry 150(4):656–657
De Souza EB (1995) Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology 20(8):789–819
del Abril A, Segovia S, Guillamon A (1987) The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Brain Res 429(2):295–300
Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG (2008) Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. Int J Dev Neurosci 26(3–4):259–268. doi:10.1016/j.ijdevneu.2008.02.004
DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK (2007) Beta-arrestins and cell signaling. Annu Rev Physiol 69:483–510. doi:10.1146/annurev.ph.69.013107.100021
Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ (2008) beta-Arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem 283(9):5669–5676. doi:10.1074/jbc.M708118200
Duncko R, Kiss A, Skultetyova I, Rusnak M, Jezova D (2001) Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology 26(1):77–89
Evuarherhe O, Leggett JD, Waite EJ, Kershaw YM, Atkinson HC, Lightman SL (2009) Organizational role for pubertal androgens on adult hypothalamic-pituitary-adrenal sensitivity to testosterone in the male rat. J Physiol 587(Pt 12):2977–2985. doi:10.1113/jphysiol.2008.168393
Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP (2007) Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab 292(4):E1173–E1182. doi:10.1152/ajpendo.00102.2006
Foote SL, Aston-Jones G, Bloom FE (1980) Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA 77:3033–3037
Friedmann B, Kindermann W (1989) Energy metabolism and regulatory hormones in women and men during endurance exercise. Eur J Appl Physiol Occup Physiol 59(1–2):1–9
Gallucci WT, Baum A, Laue L, Rabin DS, Chrousos GP, Gold PW, Kling MA (1993) Sex differences in sensitivity of the hypothalamic–pituitary–adrenal axis. Health Psychol 12(5):420–425
Garcia-Falgueras A, Pinos H, Collado P, Pasaro E, Fernandez R, Segovia S, Guillamon A (2005) The expression of brain sexual dimorphism in artificial selection of rat strains. Brain Res 1052(2):130–138. doi:10.1016/j.brainres.2005.05.066
Garcia-Falgueras A, Pinos H, Fernandez R, Collado P, Pasaro E, Segovia S, Guillamon A (2006) Sexual dimorphism in hybrids rats. Brain Res 1123(1):42–50. doi:10.1016/j.brainres.2006.09.053
Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP (2008) Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm Behav 53(1):124–130. doi:10.1016/j.yhbeh.2007.09.003
Gold PW, Chrousos GP (1999) The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians 111(1):22–34
Gold PW, Chrousos GP (2002) Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 7(3):254–275. doi:10.1038/sj.mp.4001032
Gold PW, Wong ML, Chrousos GP, Licinio J (1996) Stress system abnormalities in melancholic and atypical depression: molecular, pathophysiological, and therapeutic implications. Mol Psychiatry 1(4):257–264
Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW (2001) Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J Neurochem 76(2):509–519
Guillamon A, de Blas MR, Segovia S (1988) Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res 468(2):306–310
Haas DA, George SR (1988) Gonadal regulation of corticotropin-releasing factor immunoreactivity in hypothalamus. Brain Res Bull 20(3):361–367
Hajos-Korcsok E, Robinson DD, Yu JH, Fitch CS, Walker E, Merchant KM (2003) Rapid habituation of hippocampal serotonin and norepinephrine release and anxiety-related behaviors, but not plasma corticosterone levels, to repeated footshock stress in rats. Pharmacol Biochem Behav 74(3):609–616
Han TM, De Vries GJ (2003) Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol 54(3):502–510. doi:10.1002/neu.10157
Handa RJ, Burgess LH, Kerr JE, O’Keefe JA (1994) Gonadal steroid hormone receptors and sex differences in the hypothalamo–pituitary–adrenal axis. Horm Behav 28(4):464–476. doi:10.1006/hbeh.1994.1044
Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM (2009) Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci 1179:120–143. doi:10.1111/j.1749-6632.2009.05011.x
Heinsbroek RP, Van Haaren F, Feenstra MG, Endert E, Van de Poll NE (1991) Sex- and time-dependent changes in neurochemical and hormonal variables induced by predictable and unpredictable footshock. Physiol Behav 49(6):1251–1256
Herbst AL, Yates FE, Glenister DW, Urquhart J (1960) Variations in hepatic inactivation of corticosterone with changes in food intake: an explanation of impaired corticosteroid metabolism following noxious stimuli. Endocrinology 67:222–238
Heuser IJ, Gotthardt U, Schweiger U, Schmider J, Lammers CH, Dettling M, Holsboer F (1994) Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiol Aging 15(2):227–231
Heuser I, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Nemeroff CB, Holsboer F (1998) Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety 8(2):71–79
Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS (2006) Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem 96(4):934–949
Iteke O, Bakare MO, Agomoh AO, Uwakwe R, Onwukwe JU (2011) Road traffic accidents and posttraumatic stress disorder in an orthopedic setting in South-Eastern Nigeria: a controlled study. Scand J Trauma Resusc Emerg Med 19:39. doi:10.1186/1757-7241-19-39
Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T (2009) Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology 34(2):226–237. doi:10.1016/j.psyneuen.2008.09.003
Jedema HP, Grace AA (2004) Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci 24(43):9703–9713
Kawahara H, Kawahara Y, Westerink BH (2000) The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of norepinephrine in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur J Pharmacol 387:279–286
Kessler RC (2003) Epidemiology of women and depression. J Affect Disord 74(1):5–13
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995) Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry 52(12):1048–1060
Kirschbaum C, Wust S, Hellhammer D (1992) Consistent sex differences in cortisol responses to psychological stress. Psychosom Med 54(6):648–657
Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH (1995) Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom Med 57(1):23–31
Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH (1999) Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom Med 61(2):154–162
Kitay JI (1961) Sex differences in adrenal cortical secretion in the rat. Endocrinology 68:818–824
Koob GF (1999) Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 46(9):1167–1180
Kosoyan HP, Grigoriadis DE, Tache Y (2005) The CRF(1) receptor antagonist, NBI-35965, abolished the activation of locus coeruleus neurons induced by colorectal distension and intracisternal CRF in rats. Brain Res 1056(1):85–96. doi:10.1016/j.brainres.2005.07.010
Krupnick JG, Benovic JL (1998) The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol 38:289–319. doi:10.1146/annurev.pharmtox.38.1.289
Kudielka BM, Kirschbaum C (2005) Sex differences in HPA axis responses to stress: a review. Biol Psychol 69(1):113–132. doi:10.1016/j.biopsycho.2004.11.009
Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA (2008) Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage 41(3):1032–1043. doi:10.1016/j.neuroimage.2008.03.009
Lechner SM, Curtis AL, Brons R, Valentino RJ (1997) Locus coeruleus activation by colon distention: role of corticotropin-releasing factor and excitatory amino acids. Brain Res 756(1–2):114–124
Lefkowitz RJ (1998) G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem 273(30):18677–18680
Lefkowitz RJ, Shenoy SK (2005) Transduction of receptor signals by beta-arrestins. Science 308(5721):512–517. doi:10.1126/science.1109237
Leuner B, Shors TJ (2004) New spines, new memories. Mol Neurobiol 29(2):117–130. doi:10.1385/MN:29:2:117
Liu Z, Zhu F, Wang G, Xiao Z, Wang H, Tang J, Wang X, Qiu D, Liu W, Cao Z, Li W (2006) Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci Lett 404(3):358–362. doi:10.1016/j.neulet.2006.06.016
Livezey GT, Miller JM, Vogel WH (1985) Plasma norepinephrine, epinephrine and corticosterone stress responses to restraint in individual male and female rats, and their correlations. Neurosci Lett 62(1):51–56
Lodge DJ, Grace AA (2005) Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J Pharmacol Exp Ther 314(1):201–206. doi:10.1124/jpet.105.084913
Lund TD, Munson DJ, Haldy ME, Handa RJ (2004) Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 16(3):272–278. doi:10.1111/j.0953-8194.2004.01167.xJNE1167
Mark TL, Shern DL, Bagalman JE, Cao Z (2007) Ranking America’s mental health: an analysis of depression across the states. Copyright Mental Health America. Mental Health America, Alexandria
McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM (1998) Neonatal sex hormones have ‘organizational’ effects on the hypothalamic–pituitary–adrenal axis of male rats. Brain Res Dev Brain Res 105(2):295–307
Melia KR, Duman RS (1991) Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc Natl Acad Sci USA 88:8382–8386
Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H (2004) Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci 24(6):1478–1485
Nemeroff CB (1996) The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry 1(4):336–342
Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W (1984) Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226(4680):1342–1344
Nemeroff CB, Bissette G, Akil H, Fink M (1991) Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy. Corticotrophin-releasing factor, beta-endorphin and somatostatin. Br J Psychiatry 158:59–63
Oakley RH, Olivares-Reyes JA, Hudson CC, Flores-Vega F, Dautzenberg FM, Hauger RL (2007) Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and beta-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol Regul Integr Comp Physiol 293(1):R209–R222
O’Donnell T, Hegadoren KM, Coupland NC (2004) Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology 50(4):273–283
Olff M, Langeland W, Draijer N, Gersons BP (2007) Gender differences in posttraumatic stress disorder. Psychol Bull 133(2):183–204. doi:10.1037/0033-2909.133.2.183
Owens MJ, Nemeroff CB (1991) Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev 43(4):425–473
Owens MJ, Bissette G, Nemeroff CB (1989) Acute effects of alprazolam and adinazolam on the concentrations of corticotropin-releasing factor in the rat brain. Synapse 4(3):196–202. doi:10.1002/syn.890040304
Owens MJ, Vargas MA, Knight DL, Nemeroff CB (1991) The effects of alprazolam on corticotropin-releasing factor neurons in the rat brain: acute time course, chronic treatment and abrupt withdrawal. J Pharmacol Exp Ther 258(1):349–356
Page ME, Abercrombie ED (1999) Discrete local application of corticotropin-releasing factor increases locus coeruleus discharge and extracellular norepinephrine in rat hippocampus. Synapse 33:304–313
Page ME, Berridge CW, Foote SL, Valentino RJ (1993) Corticotropin-releasing factor in the locus coeruleus mediates EEG activation associated with hypotensive stress. Neurosci Lett 164:81–84
Paris JJ, Franco C, Sodano R, Freidenberg B, Gordis E, Anderson DA, Forsyth JP, Wulfert E, Frye CA (2010) Sex differences in salivary cortisol in response to acute stressors among healthy participants, in recreational or pathological gamblers, and in those with posttraumatic stress disorder. Horm Behav 57(1):35–45. doi:10.1016/j.yhbeh.2009.06.003
Perkonigg A, Kessler RC, Storz S, Wittchen HU (2000) Traumatic events and post-traumatic stress disorder in the community: prevalence, risk factors and comorbidity. Acta Psychiatr Scand 101(1):46–59
Premont RT (2005) Once and future signaling: G protein-coupled receptor kinase control of neuronal sensitivity. Neuromol Med 7(1–2):129–147. doi:10.1385/NMM:7:1-2:129
Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF (1994) Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology 60(4):436–444
Rassnick S, Hoffman GE, Rabin BS, Sved AF (1998) Injection of corticotropin-releasing hormone into the locus coeruleus or foot shock increases neuronal Fos expression. Neuroscience 85:259–268
Redei E, Li L, Halasz I, McGivern RF, Aird F (1994) Fast glucocorticoid feedback inhibition of ACTH secretion in the ovariectomized rat: effect of chronic estrogen and progesterone. Neuroendocrinology 60(2):113–123
Revankar CM, Vines CM, Cimino DF, Prossnitz ER (2004) Arrestins block G protein-coupled receptor-mediated apoptosis. J Biol Chem 279(23):24578–24584. doi:10.1074/jbc.M402121200M402121200
Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ (2005) Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci 22(1):93–106
Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ (2006) Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci 23(11):2991–2998
Reyes BA, Valentino RJ, Van Bockstaele EJ (2008) Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology 149(1):122–130
Rivier C (1999) Gender, sex steroids, corticotropin-releasing factor, nitric oxide, and the HPA response to stress. Pharmacol Biochem Behav 64(4):739–751
Rodaros D, Caruana DA, Amir S, Stewart J (2007) Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience 150(1):8–13. doi:10.1016/j.neuroscience.2007.09.043
Sandanger I, Nygard JF, Sorensen T, Moum T (2004) Is women’s mental health more susceptible than men’s to the influence of surrounding stress? Soc Psychiatry Psychiatr Epidemiol 39(3):177–184. doi:10.1007/s00127-004-0728-6
Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, Malaspina D (2003) Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry 54(12):1382–1388
Schulz C, Lehnert H (1996) Activation of noradrenergic neurons in the locus coeruleus by corticotropin-releasing factor, a microdialysis study. Neuroendocrinology 63:454–458
Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL (2004) Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav 45(4):242–249. doi:10.1016/j.yhbeh.2003.12.007S0018506X04000121
Schulz KM, Molenda-Figueira HA, Sisk CL (2009) Back to the future: the organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav 55(5):597–604. doi:10.1016/j.yhbeh.2009.03.010
Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL (2004) Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic–pituitary–adrenal axis activity of male and female rats. J Neuroendocrinol 16(12):989–998
Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL (2005a) Postnatal masculinization alters the HPA axis phenotype in the adult female rat. J Physiol 563(Pt 1):265–274. doi:10.1113/jphysiol.2004.078212
Seale JV, Wood SA, Atkinson HC, Lightman SL, Harbuz MS (2005b) Organizational role for testosterone and estrogen on adult hypothalamic–pituitary–adrenal axis activity in the male rat. Endocrinology 146(4):1973–1982. doi:10.1210/en.2004-1201
Seeman TE, Singer B, Wilkinson CW, McEwen B (2001) Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology 26(3):225–240
Shipley MT, Fu L, Ennis M, Liu W, Aston-Jones G (1996) Dendrites of locus coeruleus neurons extend preferentially into two pericoerulear zones. J Comp Neurol 365:56–68
Shukla AK, Xiao K, Lefkowitz RJ (2011) Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci 36(9):457–469. doi:10.1016/j.tibs.2011.06.003
Smagin GN, Zhou J, Harris RB, Ryan DH (1997) CRF receptor antagonist attenuates immobilization stress-induced norepinephrine release in the prefrontal cortex in rats. Brain Res Bull 42(6):431–434
Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR, Laird NM, Kagan J, Snidman N, Hirshfeld-Becker D, Tsuang MT, Sklar PB, Slaugenhaupt SA (2003) Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol Psychiatry 54(12):1376–1381
Snyder K, Wang WW, Han R, McFadden K, Valentino RJ (2011) Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. doi:10.1038/npp.2011.218
Southwick SM, Bremner JD, Rasmusson A, Morgan CA 3rd, Arnsten A, Charney DS (1999) Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry 46(9):1192–1204
Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, Chen A, Peeters BW, Roubos EW, Kozicz T (2011) Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS ONE 6(11):e28128. doi:10.1371/journal.pone.0028128PONE-D-11-14868
Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Roubos EW, Peeters BW, Kozicz T (2012) Sex-dependent and differential responses to acute restraint stress of corticotropin-releasing factor-producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis. J Neurosci Res 90(1):179–192. doi:10.1002/jnr.22737
Swanson LW, Hartman BK (1975) The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol 163(4):467–505. doi:10.1002/cne.901630406
Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983) Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36(3):165–186
Swerdloff RS, Wang C, Hines M, Gorski R (1992) Effect of androgens on the brain and other organs during development and aging. Psychoneuroendocrinology 17(4):375–383
Swinny JD, Valentino RJ (2006) Corticotropin-releasing factor promotes growth of brain norepinephrine neuronal processes through Rho GTPase regulators of the actin cytoskeleton in rat. Eur J Neurosci 24(9):2481–2490
Swithers SE, McCurley M, Hamilton E, Doerflinger A (2008) Influence of ovarian hormones on development of ingestive responding to alterations in fatty acid oxidation in female rats. Horm Behav 54(3):471–477. doi:10.1016/j.yhbeh.2008.05.009
Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK (2005) Regulation of corticotropin-releasing hormone receptor type 1alpha signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol 19(2):474–490
Tjoumakaris SI, Rudoy C, Peoples J, Valentino RJ, Van Bockstaele EJ (2003) Cellular interactions between axon terminals containing endogenous opioid peptides or corticotropin-releasing factor in the rat locus coeruleus and surrounding dorsal pontine tegmentum. J Comp Neurol 466(4):445–456
Tolin DF, Foa EB (2006) Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull 132(6):959–992. doi:10.1037/0033-2909.132.6.959
Troop RC (1959) Influence of gonadal hormones on the metabolism of cortisone. Endocrinology 64(5):671–675
Uhart M, Chong RY, Oswald L, Lin PI, Wand GS (2006) Gender differences in hypothalamic–pituitary–adrenal (HPA) axis reactivity. Psychoneuroendocrinology 31(5):642–652
Valentino RJ, Commons KG (2005) Peptides that fine-tune the serotonin system. Neuropeptides 39(1):1–8
Valentino RJ, Foote SL (1987) Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology 45:28–36
Valentino RJ, Foote SL (1988) Corticotropin-releasing hormone increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci 8(3):1016–1025
Valentino RJ, Van Bockstaele E (2001) Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology 158(4):331–342
Valentino RJ, Van Bockstaele E (2005) Functional interactions between stress neuromediator and the locus coeruleur–noradrenaline system. In: Steckler TK (ed) Handbook of stress and the brain. Elsevier, Amsterdam, pp 465–486
Valentino RJ, Van Bockstaele E (2008) Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol 583(2–3):194–203
Valentino RJ, Wehby RG (1988) Corticotropin-releasing factor: evidence for a neurotransmitter role in the locus ceruleus during hemodynamic stress. Neuroendocrinology 48(6):674–677
Valentino RJ, Page ME, Curtis AL (1991) Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res 555(1):25–34
Valentino RJ, Page ME, Van Bockstaele E, Aston-Jones G (1992) Corticotropin-releasing factor innervation of the locus coeruleus region: distribution of fibers and sources of input. Neuroscience 48:689–705
Valentino RJ, Chen S, Zhu Y, Aston-Jones G (1996) Evidence for divergent projections of corticotropin-releasing hormone neurons of Barrington’s nucleus to the locus coeruleus and spinal cord. Brain Res 732:1–15
Vamvakopoulos NC, Chrousos GP (1993) Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J Clin Investig 92(4):1896–1902. doi:10.1172/JCI116782
Van Bockstaele EJ, Colago EE, Valentino RJ (1996) Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol 364(3):523–534. doi:10.1002/(SICI)1096-9861(19960115)364:3<523:AID-CNE10>3.0.CO;2-Q
Van Bockstaele EJ, Colago EE, Valentino RJ (1998) Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol 10(10):743–757
Van Bockstaele EJ, Peoples J, Valentino RJ (1999) A.E. Bennett Research Award. Anatomic basis for differential regulation of the rostrolateral peri-locus coeruleus region by limbic afferents. Biol Psychiatry 46(10):1352–1363
Viau V, Bingham B, Davis J, Lee P, Wong M (2005) Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology 146(1):137–146. doi:10.1210/en.2004-0846en.2004-0846
Violin JD, Lefkowitz RJ (2007) Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28(8):416–422. doi:10.1016/j.tips.2007.06.006
Violin JD, Dewire SM, Barnes WG, Lefkowitz RJ (2006) G protein-coupled receptor kinase and beta-arrestin-mediated desensitization of the angiotensin II type 1A receptor elucidated by diacylglycerol dynamics. J Biol Chem 281(47):36411–36419. doi:10.1074/jbc.M607956200
Walker DL, Toufexis DJ, Davis M (2003) Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463(1–3):199–216
Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A (2008) Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol 586(8):2157–2170. doi:10.1113/jphysiol.2007.150078
Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF (2008) Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry 13(8):786–799, 741. doi:10.1038/mp.2008.38
Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ (2003) Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA 100(19):10782–10787. doi:10.1073/pnas.1834556100
Weinstock M, Razin M, Schorer-Apelbaum D, Men D, McCarty R (1998) Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int J Dev Neurosci 16(3–4):289–295
Weiser MJ, Handa RJ (2009) Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic–pituitary–adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience 159(2):883–895. doi:10.1016/j.neuroscience.2008.12.058
Whalen EJ, Rajagopal S, Lefkowitz RJ (2011) Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med 17(3):126–139. doi:10.1016/j.molmed.2010.11.004
Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F (2000) Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 34(3):171–181
Acknowledgments
The authors acknowledge the support of PHS Grants MH40008 to RJV and MH092438 to DAB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bangasser, D.A., Valentino, R.J. Sex Differences in Molecular and Cellular Substrates of Stress. Cell Mol Neurobiol 32, 709–723 (2012). https://doi.org/10.1007/s10571-012-9824-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-012-9824-4