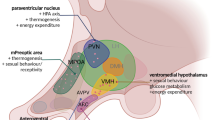
Abstract
The peripubertal period is critical for the final maturation of circuits controlling energy homeostasis and stress response. However, the consequence of juvenile fat consumption on adult physiology is not clear. This study analyzed the adult consequences of post-weaning fat feeding on limbic–hypothalamic–pituitary–adrenal (HPA) axis components and on metabolic regulators of female rats. Wistar rats were fed either a high fat (HF) diet or the normal chow from weaning to puberty or to 3 months of age. Additional groups crossed their diets at puberty onset. Plasma leptin, insulin, and corticosterone levels were determined by radioimmunoassay and their brain receptors by western blot analysis. Adult HF-fed animals though not overweight, had higher corticosterone and reduced glucocorticoid receptor levels in the hypothalamus and hippocampus, compared to the controls. The alterations in HPA axis emerged already at puberty onset. Leptin receptor levels in the hypothalamus were reduced only by continuous fat feeding from weaning to adulthood. The pre-pubertal period appeared more vulnerable to diet-induced alterations in adulthood than the post-pubertal one. Switching from fat diet to normal chow at puberty onset restored most of the diet-induced alterations in the HPA axis. The corticosteroid circuit rather than the leptin or insulin system appears as the principal target for the peripubertal fat diet-induced effects in adult female rats.




Similar content being viewed by others
References
Ahmed-Sorour H, Bailey CJ (1981) Role of ovarian hormones in the long-term control of glucose homeostasis, glycogen formation and gluconeogenesis. Ann Nutr Metab 25:208–212
Armario A, Castellanos JM, Balasch J (1985) Chronic noise stress and insulin secretion in male rats. Physiol Behav 34:359–361
Badman MK, Flier JS (2007) The adipocyte as an active participant in energy balance and metabolism. Gastroenterology 132:2103–2115
Boukouvalas G, Antoniou K, Papalexi E, Kitraki E (2008) Postweaning high fat feeding affects rats’ behavior and hypothalamic pituitary adrenal axis at the onset of puberty in a sexually dimorphic manner. Neuroscience 153:373–382
Boukouvalas G, Gerozissis K, Kitraki E (2009) Fat feeding of rats during pubertal growth leads to neuroendocrine alterations in adulthood. Cell Mol Neurobiol (Epub ahead of print) doi:10.1007/s10571-009-9434-y
Boullu-Ciocca S, Paulmyer-Lacroix O, Fina F, Ouafik L, Alessi MC, Oliver C, Grino M (2003) Expression of the mRNAs coding for the glucocorticoid receptor isoforms in obesity. Obes Res 11:925–929
Buwalda B, Blom WA, Koolhaas JM, van Dijk G (2001) Behavioral and physiological responses to stress are affected by high-fat feeding in male rats. Physiol Behav 73:371–377
De Kloet ER, Karst H, Joëls M (2008) Corticosteroid hormones in the central stress response: quick-and-slow. Frontiers Neuroendocrinol 29:268–272
Drake AJ, Livingstone DE, Andrew R, Seckl JR, Morton NM, Walker BR (2005) Reduced adipose glucocorticoid reactivation and increased hepatic glucocorticoid clearance as an early adaptation to high-fat feeding in Wistar rats. Endocrinology 146:913–999
Fehm HL, Kern W, Peters A (2006) The selfish brain: competition for energy resources. Prog Brain Res 153:129–140
Gao Q, Horvath TL (2008) Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab 294:817–826
Gerozissis K (2008) Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur J Pharmacol 585:38–49
Ghibaudi L, Cook J, Farley C, van Heek M, Hwa JJ (2002) Fat intake affects adiposity, comorbidity factors, and energy metabolism of sprague-dawley rats. Obes Res 10:956–963
Golden SH (2007) A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Curr Diabetes Rev 3:252–259
Haslam DW, James WP (2005) Obesity. Lancet 366:1197–1209
Hauner H, Schmid P, Pfeiffer EF (1987) Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J Clin Endocrinol Metab 64:832–835
Isgor C, Kabbaj M, Akil H, Watson SJ (2004) Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus 14:636–648
Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW (2000) Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes 49:1525–1533
Kamara K, Eskay R, Castonguay T (1998) High-fat diets and stress responsivity. Physiol Behav 64:1–6
Kitay JI, Coyne MD, Newsom W, Nelson R (1965) Relation of the ovary to adrenal corticosterone production and adrenal enzyme activity in the rat. Endocrinology 77:902–908
Kitraki E, Soulis G, Gerozissis K (2004) Impaired neuroendocrine response to stress following a short term fat-enriched diet. Neuroendocrinology 79:338–345
Kong AP, Chan NN, Chan JC (2006) The role of adipocytokines and neurohormonal dysregulation in metabolic syndrome. Curr Diabetes Rev 2:397–407
Leibowitz SF, Akabayashi A, Wang J, Alexander JT, Dourmashkin JT, Chang GQ (2007) Increased caloric intake on a fat-rich diet: role of ovarian steroids and galanin in the medial preoptic and paraventricular nuclei and anterior pituitary of female rats. J Neuroendocrinol 19:753–766
Leibowitz SF, Akabayashi A, Alexander J, Karatayev O, Chang GQ (2009) Puberty onset in female rats: relationship with fat intake, ovarian steroids and the peptides, galanin and enkephalin, in the paraventricular and medial preoptic nuclei. J Neuroendocrinol 21:538–549
Levin BE, Dunn-Meynell AA (2002) Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283:R941–R948
Martin B, Pearson M, Brenneman R, Golden E, Keselman A, Iyun T, Carlson OD, Egan JM, Becker KG, Wood W III, Prabhu V, de Cabo R, Maudsley S, Mattson MP (2008) Conserved and differential effects of dietary energy intake on the hippocampal transcriptomes of females and males. PLoS One 3:e2398
Mattsson C, Olsson T (2007) Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr Med Chem 14:2918–2924
McCormick CM, Mathews IZ (2007) HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav 86:220–233
Michel C, Dunn-Meynell A, Levin BE (2004) Reduced brain CRH and GR mRNA expression precedes obesity in juvenile rats bred for diet-induced obesity. Behav Brain Res 154:511–517
Pascoe WS, Smythe GA, Storlien LH (1991) Enhanced responses to stress induced by fat feedingin rats: relationship between hypothalamic noradrenaline and blood glucose. Brain Res 550:192–196
Patchev VK, Almeida OF (1996) Gonadal steroids exert facilitating and ‘buffering’ effects on glucocorticoid-mediated transcriptional regulation and corticotrophin-releasing hormone and corticosteroid receptor genes in rat brain. J Neurosci 16:7077–7084
Patchev VK, Hayashi S, Orikasa C, Almeida OF (1995) Implications of estrogen-dependent brain organization for gender differences in hypothalamo–pituitary–adrenal regulation. FASEB J 9:419–423
Plut C, Ribiere C, Giudicelli Y, Dausse JP (2003) Hypothalamic leptin receptor and signalling molecule expressions in cafeteria diet-fed rats. J Pharmacol Exp Ther 307:544–549
Priego T, Sánchez J, Oliver P, Palou A, Picó C (2007) Sex-dependent changes of hypothalamic neuropeptides in response to a prolonged high-fat diet. Genes Nutr 2:127–128
Priego T, Sánchez J, Picó C, Palou A (2008) Sex-differential expression of metabolism-related genes in response to a high-fat diet. Obesity 16:819–826
Priego T, Sánchez J, Picó C, Palou A (2009) Sex-associated differences in the leptin and ghrelin systems related with the induction of hyperphagia under high-fat diet exposure in rats. Horm Behav 55:33–40
Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P (2009) Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 150:2109–2117
Shi H, Seeley RJ, Clegg DJ (2009) Sexual differences in the control of energy homeostasis. Frontiers Neuroendocrinol 30:396–404
Siriani D, Mitsiou DJ, Alexis MN (2003) Overexpressed glucocorticoid receptor negatively regulates gene expression under conditions that favour accumulation of non-hormone-binding forms of the receptor. J Steroid Biochem Mol Biol 84:171–180
Sisk CL, Zehr JL (2005) Pubertal hormones organize the adolescent brain and behavior. Frontiers Neuroendocrinol 26:163–174
Soulis G, Kitraki E, Gerozissis K (2005) Early neuroendocrine alterations in female rats following a diet moderately enriched in fat. Cell Mol Neurobiol 25:869–880
Soulis G, Papalexi E, Kittas C, Kitraki E (2007) Early impact of a fat-enriched diet on behavioral responses of male and female rats. Behav Neurosci 121:483–490
Spinedi E, Gaillard RC (1998) A regulatory loop between the hypothalamo-pituitary-adrenal (HPA) axis and circulating leptin: a physiological role of ACTH. Endocrinology 139:4016–4020
Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ (1997) High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol 273:E1168–E1177
Van Schothorst EM, Bunschoten A, Schrauwen P, Mensink RP, Keijer J (2009) Effects of a high-fat, low-versus high-glycemic index diet: retardation of insulin resistance involves adipose tissue modulation. FASEB J 23:1092–1101
Vegiopoulos A, Herzig S (2007) Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol 275:43–61
Wang S, Matthews SG, Yang K, Challis JR (1998) The effect of estradiol on output of adrenocorticotropin and prolactin by fetal sheep anterior pituitary cells. Can J Physiol Pharmacol 76:843–849
Weiser MJ, Handa RJ (2009) Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience 159:883–895
Woods SC (1991) The eating paradox: how we tolerate food. Psychol Rev 98:488–505
Zhang Y, Scarpace PJ (2006) The role of leptin in leptin resistance and obesity. Physiol Behav 88:249–256
Acknowledgments
This study is part of the 03ED81 research project, implemented within the framework of the “Reinforcement Program of Human Research Manpower” (PENED) that is co-financed by E.U.-European Social Fund (75%) and the Greek Ministry of Development-GSRT (25%). IASO Hospital is acknowledged for material supply and indirect financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boukouvalas, G., Gerozissis, K. & Kitraki, E. Adult Consequences of Post-weaning High Fat Feeding on the Limbic–HPA Axis of Female Rats. Cell Mol Neurobiol 30, 521–530 (2010). https://doi.org/10.1007/s10571-009-9476-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9476-1