Abstract
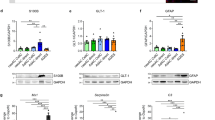
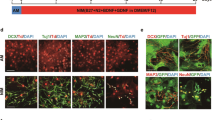
Our previous study definitely demonstrated that the mature astrocytes could undergo a de-differentiation process and further transform into pluripotential neural stem cells (NSCs), which might well arise from the effect of diffusible factors released from scratch-insulted astrocytes. However, these neurospheres passaged from one neurosphere-derived from de-differentiated astrocytes possessed a completely distinct characteristic in the differentiation behavior, namely heterogeneity of differentiation. The heterogeneity in cell differentiation has become a crucial but elusive issue. In this study, we show that purified astrocytes could de-differentiate into intermediate precursor cells (IPCs) with addition of scratch-insulted astrocyte-conditioned medium (ACM) to the culture, which can express NG2 and A2B5, the IPCs markers. Apart from the number of NG2+ and A2B5+ cells, the percentage of proliferative cells as labeled with BrdU progressively increased with prolonged culture period ranging from 1 to 10 days. Meanwhile, the protein level of A2B5 in cells also increased significantly. These results revealed that not all astrocytes could de-differentiate fully into NSCs directly when induced by ACM, rather they generated intermediate or more restricted precursor cells that might undergo progressive de-differentiation to generate NSCs.



Similar content being viewed by others
References
Abney ER, Williams BP, Raff MC (1983) Tracing the development of oligodendrocytes from precursor cells using monoclonal antibodies, fluorescenceactivated cell sorting, and cell culture. Dev Biol 100:166–171
Aguirre A, Gallo V (2004) Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci 24:10530–10541
Aguirre AA, Chittajallu R, Belachew S, Gallo V (2004) NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol 165:575–589
Baracskay KL, Kidd GJ, Miller RH, Trapp BD (2007) NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia 55:1001–1098
Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V (2003) Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol 161:169–186
Cai S, Fu XB, Sheng ZY (2007) Dedifferentiation: a new approach in stem cell research. Bioscience 57:655–662
Cenci di Bello I, Dawson MRL, Levine JM, Reynolds R (1999) Generation of oligodendroglial progenitors in acute inflammatory demyelinating lesions of the rat brain stem is associated with demyelination rather than inflammation. J Neurocytol 28:365–381
Chatterjee N, Stegmüller J, Schätzle P, Karram K, Koroll M, Werner HB, Nave KA, Trotter J (2008) Interaction of syntenin-1 and the NG2 proteoglycan in migratory oligodendrocyte precursor cells. J Biol Chem 283:8310–8317
Chittajallu R, Aguirre A, Gallo V (2004) NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol 561:109–122
Dawson M, Levine JM, Reynolds R (2000) NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res 61:471–479
Diers-Fenger M, Kirchhoff F, Kettenmann H, Levine JM, Trotter J (2001) AN2/NG2 protein expressing glial progenitor cells in the murine CNS: isolation, differentiation, and association with radial glia. Glia 34:213–228
Dromard C, Bartolami S, Deleyrolle L, Takebayashi H, Ripoll C, Simonneau L, Prome S, Puech S, Tran VB, Duperray C, Valmier J, Privat A, Hugnot JP (2007) NG2 and Olig2 expression provides evidence for phenotypic deregulation of cultured central nervous system and peripheral nervous system neural precursor cells. Stem Cells 25:340–353
Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM (2006) Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci 26:8609–8621
Grinspan J (2002) Cells and signaling in oligodendrocyte development. J Neuropathol Exp Neurol 61:297–306
Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC (2004) The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J 23:3061–3071
Keirstead HS, Levine JM, Blakemore WF (1998) Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia 22:161–170
Lang B, Liu HL, Liu R, Feng GD, Jiao XY, Ju G (2004) Astrocytes in injured adult rat spinal cord may acquire the potential of neural stem cells. Neuroscience 128:775–783
Levine JM, Reynolds R (1999) Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp Neurol 160:333–347
Levine JM, Reynolds R, Fawcett JW (2001) The oligodendrocyte precursor cell in health and disease. Trends Neurosci 24:39–47
Liu Y, Rao MS (2004) Glial progenitors in the CNS and possible lineage relationships among them. Biol Cell 96:279–290
Liu Y, Wu Y, Lee JC, Xue H, Pevny LH, Kaprielian Z, Rao MS (2002) Oligodendrocyte and astrocyte development in rodents: an in situ and immunohistological analysis during embryonic development. Glia 40:25–43
Mallon BS, Shick HE, Kidd GJ, Macklin WB (2002) Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci 22:876–885
Nishiyama A, Yu M, Drazba JA, Tuohy VK (1997) Normal and reactive NG2+ glial cells are distinct from resting and activated microglia. J Neurosci Res 48:299–312
Polito A, Reynolds R (2005) NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat 207:707–716
Rao MS, Noble M, Mayer-Proschel M (1998) A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci USA 95:3996–4001
Ruffini F, Arbour N, Blain M, Olivier A, Antel JP (2004) Distinctive properties of human adult brain-derived myelin progenitor cells. Am J Pathol 165:2167–2175
Stallcup WB (2002) The NG2 proteoglycan: past insights and future prospects. J Neurocytol 31:423–435
Stoscheck CM (1990) Quantitation of protein. Methods Enzymol 182:50–68
Walder S, Zhang F, Ferretti P (2003) Up-regulation of neural stem cell markers suggests the occurrence of dedifferentiation in regenerating spinal cord. Dev Genes Evol 213:625–630
Yang H, Liang Z, Li JW, Cheng XP, Luo N, Ju G (2006) Optimized and efficient preparation of astrocyte cultures from rat spinal cord. Cytotechnology 52:87–97
Yang H, Cheng XP, Qin Y, Li JW, Ju G (2008) The promotive effects of thymosin β4 on neuronal survival and neurite outgrowth by upregulating L1 expression. Neurochem Res 33:1169–2280
Yang H, Cheng XP, Li JW, Qin Y, Ju G (2009) De-differentiation response of cultured astrocytes to injury induced by scratch or conditioned culture medium of scratch-insulted astrocytes. Cell Mol Neurobiol 29:455–473
Acknowledgments
This work was supported by the Natural Science Foundation of China (Grant Nos. 30973088, 30872829, and 30571998), Chinese PLA national scientific technological project (06G089), and the 11th Five-year Special-purpose Programme for PLA (08Z028).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hao Yang and Xin-Hong Qian contributed equally to this article.
Rights and permissions
About this article
Cite this article
Yang, H., Qian, XH., Cong, R. et al. Evidence for Heterogeneity of Astrocyte De-Differentiation in vitro: Astrocytes Transform into Intermediate Precursor Cells Following Induction of ACM from Scratch-Insulted Astrocytes. Cell Mol Neurobiol 30, 483–491 (2010). https://doi.org/10.1007/s10571-009-9474-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9474-3