1. Several lines of evidence suggest that cytokines and their receptors are initiators of changes in the activity of dorsal root ganglia (DRG) neurons, but their cellular distribution is still very limited or controversial. Therefore, the goal of present study was to investigate immunohistochemical distribution of TNF-α and TNF receptor-1 (TNFR1) proteins in the rat DRG following three types of nerve injury.
2. The unilateral sciatic and spinal nerve ligation as well as the sciatic nerve transection were used to induce changes in the distribution of TNF-α and TNFR1 proteins. The TNF-α and TNFR1 immunofluorescence was assessed in the L4-L5 DRG affected by nerve injury for 1 and 2 weeks, and compared with the contralateral ones and those removed from naive or sham-operated rats. A part of the sections was incubated for simultaneous immunostaining for TNF-α and ED-1. The immunofluorescence brightness was measured by image analysis system (LUCIA-G v4.21) to quantify immunostaining for TNF-α and TNFR1 in the naive, ipsi- and contralateral DRG following nerve injury.
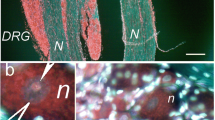
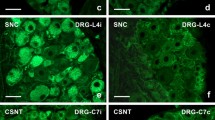
3. The ipsilateral L4-L5 DRG and their contralateral counterparts of the rats operated for nerve injury displayed an increased immunofluorescence (IF) for TNF-α and TNFR1 when compared with DRG harvested from naive or sham-operated rats. The TNFα IF was increased bilaterally in the satellite glial cells (SGC) and contralaterally in the neuronal nuclei following sciatic and spinal nerve ligature. The neuronal bodies and their SGC exhibited bilaterally enhanced IF for TNF-α after sciatic nerve transection for 1 and 2 weeks. In addition, the affected DRG were invaded by ED-1 positive macrophages which displayed simultaneously TNFα IF. The ED-1 positive macrophages were frequently located near the neuronal bodies to occupy a position of the satellites.
4. The sciatic and spinal nerve ligature resulted in an increased TNFR1 IF in the neuronal bodies of both ipsi- and contralateral DRG. The sciatic nerve ligature for 1 week induced a rise in TNFR1 IF in the contralateral DRG neurons and their SGC to a higher level than in the ipsilateral ones. In contrast, the sciatic nerve ligature for 2 weeks caused a similar increase of TNFR1 IF in the neurons and their SGC of both ipsi- and contralateral DRG. The spinal nerve ligature or sciatic nerve transection resulted in an increased TNFR1 IF located at the surface of the ipsilateral DRG neurons, but dispersed IF in the contralateral ones. In addition, the SGC of the contralateral in contrast to ipsilateral DRG displayed a higher TNFR1 IF.
5. Our results suggest more sources of TNF-α protein in the ipsilateral and contralateral DRG following unilateral nerve injury including macrophages, SGC and primary sensory neurons. In addition, the SGC and macrophages, which became to be satellites, are well positioned to regulate activity of the DRG neurons by production of TNF-α molecules. Moreover, the different cellular distribution of TNFR1 in the ipsi- and contralateral DRG may reflect different pathways by which TNF-α effect on the primary sensory neurons can be mediated following nerve injury.



Similar content being viewed by others
REFERENCES
Bennett, G. J., and Xie, Y. K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107.
Clatworthy, A. L., Illich, P. A., Castro, G. A., and Walters, E. T. (1995). Role of periaxonal inflammation in the development of thermal hyperalgesia and guarding behavior in a rat model of neuropathic pain. Neurosci. Lett. 184:5–8.
Cui, J. G., Holmin, S., Mathiesen, T., Meyerson, B. A., and Linderoth, B. (2000). Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain 88:239–248.
DeLeo, J. A., and Yezierski, R. P. (2001). The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 90:1–6.
Dinarello, C. A. (1994). The interleukin-1 family: 10 years of discovery. FASEB J. 8:1314–1325.
Dubovy, P., Svizenska, I., and Klusakova, I. (2002). Computer-assisted quantitative analysis of immunofluorescence staining of the extracellular matrix in rat dorsal and ventral spinal roots. Acta Histochem. 104:371–374.
Dubovy, P., Jancalek, R., and Klusakova, I. (2005). A heterogeneous immunofluorescence staining for laminin-1 and related basal lamina molecules in the dorsal root ganglia following constriction nerve injury. Histochem. Cell Biol. DOI 10.1007/s00418–005-0115-8.
Hu, P., and McLachlan, E. M. (2002). Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience 112:23–38.
Kim, S. H., and Chung, J. M. (1992). An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50:355–363.
Kleinschnitz, C., Brinkhoff, J., Sommer, C., and Stoll, G. (2005). Contralateral cytokine gene induction after peripheral nerve lesions: dependence on the mode of injury and NMDA receptor signaling. Mol. Brain Res. 136:23–28.
Koltzenburg, M., Wall, P. D., and McMahon, S. B. (1999). Does the right side know what the left is doing? TINS 22:122–127.
Li, Y., Ji, A., Weihe, E., and Schafer, M. K. H. (2004). Cell-Specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor α (TNF-α) and TNF receptors in rat dorsal root ganglion. J. Neurosci. 24:9623–9631.
Michaelis, M., Vogel, C., Blenk, K. H., Arnarson, A., and Janig, W. (1998). Inflammatory mediators sensitize acutely axotomized nerve fibers to mechanical stimulation in the rat. J. Neurosci. 18:7581–7587.
Murphy, P., Grondin, J., Altares, M., and Richardson, P. (1995). Induction of interleukin-6 in axotomized sensory neurons. J. Neurosci. 15:5130–5138.
Myers, R. R., Wagner, R., and Sorkin, L. S. (1999). Hyperalgesic actions of cytokines on peripheral nerves. In Watkins, L. R., and Maier, S. F. (eds.), Cytokines and Pain, Birkhauser Verlag, Basel, pp. 133–157.
Ruohonen, S., Jagodi, M., Khademi, M., Taskinen, H. S., Ojala, P., Olsson, T., and Roytta, M. (2002). Contralateral non-operated nerve to transected rat sciatic nerve shows increased expression of IL-1[beta], TGF-[beta]1, TNF-[alpha], and IL-10. J. Neuroimmunol. 132: 11–17.
Ryoke, K., Ochi, M., Iwata, A., Uchio, Y., Yamamoto, S., and Yamaguchi, H. (2000). A conditioning lesion promotes in vivo nerve regeneration in the contralateral sciatic nerve of rats. Biochem. Biophys. Res. Comm. 267:715–718.
Schafers, M., Geis, C., Brors, D., Yaksh, T. L., and Sommer, C. (2002). Anterograde transport of tumor necrosis factor-α in the intact and injured rat sciatic nerve. J. Neurosci. 2:536–545.
Schafers, M., Lee, D. H., Brors, D., Yaksh, T. L., and Sorkin, L. S. (2003a). Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J. Neurosci. 23:3028–3038.
Schafers, M., Sorkin, L. S., Geis, C., and Shubayev, V. I. (2003b). Spinal nerve ligation induces transient upregulation of tumor necrosis factor receptors 1 and 2 in injured and adjacent uninjured dorsal root ganglia in the rat. Neurosci. Lett. 347:179–182.
Schafers, M., Geis, C., Svensson, C. I., Luo, Z. D., and Sommer, C. (2003c). Selective increase of tumor necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur. J. Neurosci. 17:791–804.
Schnell, L., Fearn, S., Schwab, M. E., Perry, V. H., and Anthony, D. C. (1999). Cytokine-induced acute inflammation in the brain and spinal cord. J. Neuropathol. Exp. Neurol. 58:245–254.
Seltzer, Z., Dubner, R., and Shir, Y. (1990). A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43:205–218.
Shafer, D. M., Assael, L., White, L. B., and Rossomando, E. F. (1994). Tumor necrosis factor-alpha as a biochemical marker of pain and outcome in temporomandibular joints with internal derangements. J. Oral Maxillofac. Surg. 52:786–791.
Shubayev, V. I., and Myers, R. R. (2002). Anterograde TNF-alpha transport from rat dorsal root ganglion to spinal cord and injured sciatic nerve. Neurosci. Lett. 320:99–101.
Sommer, C., and Schafers, M. (1998). Painful mononeuropathy in C57BL/Wld mice with delayed Wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 784:154–162.
Sommer, C., Schmidt, C., and George, A. (1998). Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp. Neurol. 151:138–142.
Sorkin, L. S., Xiao, W. H., Wagner, R., and Myers, R. R. (1997). Tumor necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience 81:255–262.
Sorkin, L. S., and Doom, C. M. (2000). Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J. Periph. Nerv. Syst. 5:96–100.
Suzuki, R., and Dickenson, A. H. (2000). Neuropathic pain: nerves bursting with excitement. Neuroreport 11:R17–21.
Svensson, C. I., Schafers, M., Jones, T. L., Powell, H., and Sorkin, L. S. (2005). Spinal blockade of TNF blocks spinal nerve ligation-induced increases in spinal P-p38. Neurosci. Lett. 379:209–213.
Sweitzer, S., Martin, D., and Deleo, J. A. (2001). Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience 103:529–539.
Vandenabeele, P., Declercq, W., Beyaert, R., and Fiers, W. (1995). Two tumor necrosis factor receptors: structure and function. Trends Cell Biol. 5:392–399.
Wagner, R., and Myers, R. R. (1996a). Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport 7:2897–2901.
Wagner, R., and Myers, R. R. (1996b). Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience 73:625–629.
Wesemann, D. R., and Benveniste, E. N. (2003). STAT-1 alpha and IFN-gamma as modulators of TNF-alpha signaling in macrophages: regulation and functional implications of the TNF receptor 1:STAT-1 alpha complex. J. Immunol. 171:5313–5329.
Watkins, L. R., Wiertelak, E. P., Goehler, L. E., Smith, K. P., Martin, D., and Maier, S. F. (1994). Characterization of cytokine-induced hyperalgesia. Brain Res. 654:15–26.
Watkins, L. R., Maier, S. F., and Goehler, L. E. (1995). Immune activation: the role of proinflammatory cytokines in inflammation, illness responses and pathological pain states. Pain 63:289–302.
Woolf, C. J., and Mannion, R. J. (1999). Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 353:1959–1964.
Woolf, C. J., and Salter, M. W. (2000). Neuronal plasticity-increasing the gain in pain. Science 288:1765–1768.
Woolf, C. J., Allchorne, A., Safieh-Garabedian, B., and Poole, S. (1997). Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumor necrosis factor alpha. Br. J. Pharmacol. 121:417–424.
Xian, C. J., and Zhou, X. F. (1999). Neuronal-glial differential expression of TGF-[alpha] and its receptor in the dorsal root ganglia in response to sciatic nerve lesion. Exp. Neurol. 157:317–326.
Zamboni, L., and DeMartino, C. (1967). Buffered picric acid-formaldehyde: a new, rapid fixative for electron microscopy. J. Cell Biol. 35:148.
Zhang, J. M., Li, H., Liu, B., Brull, S. J. (2002). Acute topical application of tumor necrosis factor-alpha evokes protein kinase A-dependent responses in rat sensory neurons. J. Neurophysiol. 88:1387–1392.
Zimmermann, M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110.
Zimmermann, M. (2001). Pathobiology of neuropathic pain. Eur. J. Pharmacol. 429:23–37.
ACKNOWLEDGMENTS
We thank Ms. Dana Kutějová, Mrs. Jitka Pokorná, and Ms. Stana Bartová for their skilful technical assistance. This work was supported by grants GAČR 309/03/1199 and MSM0021622404.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubový, P., Jančálek, R., Klusáková, I. et al. Intra- and Extraneuronal Changes of Immunofluorescence Staining for TNF- and TNFR1 in the Dorsal Root Ganglia of Rat Peripheral Neuropathic Pain Models. Cell Mol Neurobiol 26, 1203–1215 (2006). https://doi.org/10.1007/s10571-006-9006-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-006-9006-3