Abstract
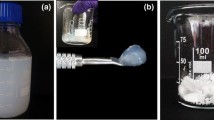
Nanocellulose gels were prepared by a new chemical crosslinking approach. Carboxy group containing cellulose nanofibrils, which were prepared by (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl (TEMPO) mediated oxidation, were reacted with two different diiodoalkanes and one triiodoalkane in DMSO/water dispersions at elevated temperatures. Mechanically stable gels were obtained that were characterized with respect to chemical and physical properties. It was confirmed by FTIR spectroscopy that crosslinking of TEMPO oxidized cellulose nanofibrils (TCNF) occurs by the formation of ester bonds. The kinetics of gel formation were evaluated by oscillatory rheology experiments. For long alkyl chain cross-linkers, namely 1,4-diiodobutane and 1,10-diiododecane, the initial gel was formed within a short time (gel point < 5 min) and further evolved upon time for about 0.5–2 h. For the short crosslinker triiodomethane, gel formation was slower and resulted in lower mechanical strength.
Graphic abstract








Similar content being viewed by others
References
Abdul Khalil HPS, Bhat AH, Ireana Yusra AF (2012) Green composites from sustainable cellulose nanofibrils: a review. Carbohyd Polym 87:963–979
Abe K, Yano H (2012) Cellulose nanofiber-based hydrogels with high mechanical strength. Cellulose 19:1907–1912
Afsahi G, Dimic-Misic K, Gane P, Budtova T, Maloney T, Vuorinen T (2018) The investigation of rheological and strength properties of NFC hydrogels and aerogels from hardwood pulp by short catalytic bleaching (Hcat). Cellulose 25:1637–1655
Aitomäki Y, Oksman K (2014) Reinforcing efficiency of nanocellulose in polymers. React Funct Polym 85:151–156
Besbes I, Alila S, Boufi S (2011) Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: effect of the carboxyl content. Carbohyd Polym 84:975–983
Brinchi L, Germani R, Savelli G (2003) Efficient esterification of carboxylic acids with alkyl halides catalyzed by fluoride ions in ionic liquids. Tetrahedron Lett 44:6583–6585
Buwalda SJ, Vermonden T, Hennink WE (2017) Hydrogels for therapeutic delivery: current developments and future directions. Biomacromol 18:316–330
Chinga-Carrasco G, Syverud K (2014) Pretreatment-dependent surface chemistry of wood nanocellulose for pH-sensitive hydrogels. J Biomater Appl 29:423–432
Coates J (2006) Interpretation of infrared spectra, a practical approach. In: Meyers RA (ed) Encyclopedia of analytical chemistry. Wiley, Chichester, pp 10815–10837
Cox BG (2015) Acids, bases, and salts in mixed-aqueous solvents. Org Process Res Dev 19:1800–1808
De France KJ, Hoare T, Cranston ED (2017) Review of hydrogels and aerogels containing nanocellulose. Chem Mater 29:4609–4631
Deng S, Huang R, Zhou M, Chen F, Fu Q (2016) Hydrophobic cellulose films with excellent strength and toughness via ball milling activated acylation of microfibrillated cellulose. Carbohydr Polym 154:129–138
Dong H, Snyder JF, Tran DT, Leadore JL (2013a) Hydrogel, aerogel and film of cellulose nanofibrils functionalized with silver nanoparticles. Carbohydr Polym 95:760–767
Dong H, Snyder JF, Williams KS, Andzelm JW (2013b) Cation-induced hydrogels of cellulose nanofibrils with tunable moduli. Biomacromol 14:3338–3345
Donius AE, Liu A, Berglund LA, Wegst UG (2014) Superior mechanical performance of highly porous, anisotropic nanocellulose-montmorillonite aerogels prepared by freeze casting. J Mech Behav Biomed Mater 37:88–99
Eyley S, Thielemans W (2014) Surface modification of cellulose nanocrystals. Nanoscale 6:7764–7779
Fujisawa S, Saito T, Kimura S, Iwata T, Isogai A (2013) Surface engineering of ultrafine cellulose nanofibrils toward polymer nanocomposite materials. Biomacromol 14:1541–1546
Heinze T, El Seoud OA, Koschella A (2018) Cellulose derivatives: synthesis, structure and properties. In: Heinze T, El Seoud OA, Koschella A (eds) Cellulose derivatives: synthesis, structure, and properties. Springer, Cham, pp 429–477
Hennis HE, Easterly JP, Collins LR, Thompson LR (1967) Esters from reactions of alkyl halides and salts of carboxylic acids. Reactions of primary alkyl chlorides and sodium Salts of carboxylic acids. I&EC Prod Res Dev 6:193–195
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3:71–85
Jausovec D, Vogrincic R, Kokol V (2015) Introduction of aldehyde vs. carboxylic groups to cellulose nanofibers using laccase/TEMPO mediated oxidation. Carbohydr Polym 116:74–85
Jiang F, Han S, Hsieh Y-L (2013) Controlled defibrillation of rice straw cellulose and self-assembly of cellulose nanofibrils into highly crystalline fibrous materials. RSC Adv 3:12366
Liu J, Chinga-Carrasco G, Cheng F, Xu W, Willför S, Syverud K, Xu C (2016) Hemicellulose-reinforced nanocellulose hydrogels for wound healing application. Cellulose 23:3129–3143
Liu M et al (2017a) Injectable hydrogels for cartilage and bone tissue engineering. Bone Res 5:17014
Liu P, Mai C, Zhang K (2017b) Formation of uniform multi-stimuli-responsive and multiblock hydrogels from dialdehyde cellulose. ACS Sustain Chem Eng 5:5313–5319
Marcus Y (2007) Preferential solvation of ions in mixed solvents. 5. The alkali metal, silver, and thallium(I) cations in aqueous organic solvents according to the inverse Kirkwood–Buff integral (IKBI) approach. J Sol Chem 36:1385–1399
Matsumoto K et al (2014) Simple and convenient synthesis of esters from carboxylic acids and alkyl halides using tetrabutylammonium fluoride. J Oleo Sci 63:539–544
Naderi A, Lindström T, Sundström J (2014) Carboxymethylated nanofibrillated cellulose: rheological studies. Cellulose 21:1561–1571
Naderi A, Lindström T, Sundström J, Pettersson T, Flodberg G, Erlandsson J (2015) Microfluidized carboxymethyl cellulose modified pulp: a nanofibrillated cellulose system with some attractive properties. Cellulose 22:1159–1173
Nicholson DJ, Leavitt AT, Francis RC (2014) A three stage Klason method for more accurate determinations of hardwood lignin content. Cell Chem Technol 48:53–59
Nordenstrom M, Fall A, Nystrom G, Wagberg L (2017) Formation of colloidal nanocellulose glasses and gels. Langmuir 33:9772–9780
Okita Y, Fujisawa S, Saito T, Isogai A (2011) TEMPO-oxidized cellulose nanofibrils dispersed in organic solvents. Biomacromol 12:518–522
Park M, Lee D, Hyun J (2015) Nanocellulose-alginate hydrogel for cell encapsulation. Carbohydr Polym 116:223–228
Phaodee P, Tangjaroensirirat N, Sakdaronnarong C (2014) Biobased polystyrene foam-like material from crosslinked cassava starch and nanocellulose from sugarcane. BioResources 10:348–368
Philipp B, Schleicher H, Wagenknecht W (1973) The influence of cellulose structure on the swelling of cellulose in organic liquids. J Polym Sci Polym Symp 42:1531–1543
Puls J, Janzon R, Saake B (2006) Comparative removal of hemicelluloses from paper pulps using Nitren, Cuen, NaOH and KOH. Lenzinger Ber 86:63–70
Rol F, Belgacem MN, Gandini A, Bras J (2019) Recent advances in surface-modified cellulose nanofibrils. Progr Polym Sci 88:241–264
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromol 8:2485–2491
Saito T, Uematsu T, Kimura S, Enomae T, Isogai A (2011) Self-aligned integration of native cellulose nanofibrils towards producing diverse bulk materials. Soft Matter 7:8804–8809
Sehaqui H, Zhou Q, Berglund LA (2011) High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Comp Sci Technol 71:1593–1599
Syverud K, Pettersen SR, Draget K, Chinga-Carrasco G (2014) Controlling the elastic modulus of cellulose nanofibril hydrogels scaffolds with potential in tissue engineering. Cellulose 22:473–481
Thomas B et al (2018) Nanocellulose, a versatile green platform: from biosources to materials and their applications. Chem Rev 118:11575–11625
Turbak AF, Snyder FW, Sandberg KR (1983) Microfibrillated cellulose, a new cellulose product: properties, uses, and commercial potential. J Appl Polym Sci Appl Polym Symp USA. In: Cellulose conference, Syracuse, NY, USA, 24 May 1982.; ITT Rayonier Inc., Shelton
Wågberg L, Decher G, Norgren M, Lindström T, Ankerfors M, Axnäs K (2008) The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 24:784–795
Wang W, Li F, Yu J, Navard P, Budtova T (2015) Influence of substitution on the rheological properties and gelation of hydroxyethyl cellulose solution in NaOH–water solvent. Carbohydr Polym 124:85–89
Zander NE, Dong H, Steele J, Grant JT (2014) Metal cation cross-linked nanocellulose hydrogels as tissue engineering substrates. ACS Appl Mater Interfaces 6:18502–18510
Acknowledgments
We thank our colleagues from Domsjö fabriker AB (Sweden) for supplying the never dried sulfite pulp on which this experiment was conducted. The authors thank the Slovenian Research Agency within the research program P4-0015 and the Slovene science foundation for their financial support. The authors also acknowledge the Ministry of Education, Science and Sport of the Republic of Slovenia for financial support of the “young scientist” programme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Levanič, J., Gericke, M., Heinze, T. et al. Stable nanocellulose gels prepared by crosslinking of surface charged cellulose nanofibrils with di- and triiodoalkanes. Cellulose 27, 2053–2068 (2020). https://doi.org/10.1007/s10570-019-02947-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02947-3