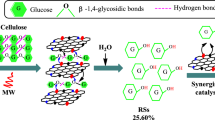
Abstract
The regenerated solid acid (Cl-MCMB-SO3H) catalyst containing bifunctional groups, namely, –SO3H and –Cl, was prepared using mesocarbon microbeads (MCMB) with numerous aromatic constituents. The acidic densities of –SO3H and –Cl in the as-prepared Cl-MCMB-SO3H reached 1.98 and 1.72 mmol/g, respectively. The total reducing sugar (TRS) yield of 70.3% was achieved at 130 °C for 3 h in distilled water with Cl-MCMB-SO3H solid acid as a catalyst. The analysis of hydrolytic kinetic model showed that the activation energy (Ea) of cellulose hydrolysis by using Cl-MCMB-SO3H was 89.4 kJ/mol, which was considerably lower than that of MCMB-SO3H (109.2 kJ/mol). Thus, the existence of –Cl group was not only beneficial in forming hydrogen bonds with cellulose but also aided in reducing the Ea value of cellulose hydrolysis. The TRS yield only decreased by 9.6% with Cl-MCMB-SO3H solid acid after it was recycled five times. More importantly, the TRS yield still reached 70.1% and 69.7% through the first and second Cl-MCMB-SO3H regeneration, respectively, demonstrating the excellent regenerability of the solid acid catalyst.
Graphic abstract
The multifunctional MCMB-solid acid was prepared and used for the catalytic hydrolysis of cellulose, which exhibited excellent catalysis, regenerability, and recyclability performances, and the introduction of –Cl group not only facilitated the formation of hydrogen bonds with cellulose but also dramatically decreased the activation energy.









Similar content being viewed by others
References
Akin O, Yuksel A (2016) Novel hybrid process for the conversion of microcrystalline cellulose to value-added chemicals: part 1: process optimization. Cellulose 23:3475–3493
Akin O, Yuksel A (2017) Novel hybrid process for the conversion of microcrystalline cellulose to value-added chemicals: part 2: effect of constant voltage on product selectivity. Cellulose 24:4729–4741
Alonso DM, Bond JQ, Dumesic JA (2010) Catalytic conversion of biomass to biofuels. Green Chem 12:1493–1513
Bai C, Zhu L, Shen F, Qi X (2016) Black liquor-derived carbonaceous solid acid catalyst for the hydrolysis of pretreated rice straw in ionic liquid. Bioresour Technol 220:656–660
Cantero DA, Bermejo MD, Cocero MJ (2015) Governing chemistry of cellulose hydrolysis in supercritical water. Chemsuschem 8:1026–1033
Chen Y, Ai X, Huang B, Huang M, Huang Y, Lu Y (2017) Consecutive preparation of hydrochar catalyst functionalized in situ with sulfonic groups for efficient cellulose hydrolysis. Cellulose 24:2743–2752
Cheng J, Qiu Y, Zhang J, Huang R, Yang W, Fan Z (2017) Conversion of lipids from wet microalgae into biodiesel using sulfonated graphene oxide catalysts. Bioresour Technol 244:569–574
de Oliveira Ribeiro WC, da Silva Lima AC, de Araújo Morandim-Giannetti A (2018) Optimizing treatment condition of coir fiber with ionic liquid and subsequent enzymatic hydrolysis for future bioethanol production. Cellulose 25:527–536
Dong K, Zhang J, Luo W, Su L, Huang Z (2018) Catalytic conversion of carbohydrates into 5-hydroxymethyl furfural over sulfonated hyper-cross-linked polymer in DMSO. Chem Eng J 334:1055–1064
Fontanals N, Marcé RM, Borrull F, Cormack PAG (2015) Hypercrosslinked materials: preparation, characterisation and applications. Polym Chem 6:7231–7244
Gan L, Zhu J, Lv L (2017) Cellulose hydrolysis catalyzed by highly acidic lignin-derived carbonaceous catalyst synthesized via hydrothermal carbonization. Cellulose 24:5327–5339
Gao Y, Song H, Chen X (2003) Self-sinterability of mesocarbon microbeads (MCMB) for preparation of high-density isotropic carbon. J Mater Sci 38:2209–2213
Goswami M, Meena S, Navatha S, Prasanna Rani KN, Pandey A, Sukumaran RK, Prasad RB, Prabhavathi Devi BL (2015) Hydrolysis of biomass using a reusable solid carbon acid catalyst and fermentation of the catalytic hydrolysate to ethanol. Bioresour Technol 188:99–102
Hu S, Smith TJ, Lou W, Zong M (2014) Efficient hydrolysis of cellulose over a novel sucralose-derived solid acid with cellulose-binding and catalytic sites. J Agric Food Chem 62:1905–1911
Hu L, Li Z, Wu Z, Lin L, Zhou S (2016) Catalytic hydrolysis of microcrystalline and rice straw-derived cellulose over a chlorine-doped magnetic carbonaceous solid acid. Ind Crop Prod 84:408–417
Huang Y, Fu Y (2013) Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem 15:1095–1111
Jiang L, Wu N, Zheng A, Liu A, Zhao Z, Zhang F, He F, Li H (2017) Comprehensive utilization of hemicellulose and cellulose to release fermentable sugars from corncobs via acid hydrolysis and fast pyrolysis. ACS Sustain Chem Eng 5:5208–5213
Konwar LJ, Mäki-Arvela P, Salminen E, Kumar N, Thakur AJ, Mikkola J-P, Deka D (2015) Towards carbon efficient biorefining: Multifunctional mesoporous solid acids obtained from biodiesel production wastes for biomass conversion. Appl Catal B Environ 176:20–35
Konwar LJ, Samikannu A, Mäki-Arvela P, Boström D, Mikkola J-P (2018) Lignosulfonate-based macro/mesoporous solid protonic acids for acetalization of glycerol to bio-additives. Appl Catal B Environ 220:314–323
Li C, Wang Q, Zhao ZK (2008) Acid in ionic liquid: an efficient system for hydrolysis of lignocellulose. Green Chem 10:177–182
Li T, Shen S, Cai B, Wang Y, Peng X, Li Y (2016) High-performance carbon-based solid acid prepared by environmental and efficient recycling of PVC waste for cellulose hydrolysis. RSC Adv 6:91921–91929
Li H, Xu Z, Yan P, Zhang ZC (2017) A catalytic aldol condensation system enables one pot conversion of biomass saccharides to biofuel intermediates. Green Chem 19:1751–1756
Li HX, Zhang K, Zhang X, Cao Q, Le Jin (2018a) Contributions of ultrasonic wave, metal ions, and oxidation on the depolymerization of cellulose and its kinetics. Renew Energy 126:699–707
Li HX, Zhang X, Wang Q, Zhang K, Cao Q, Le Jin (2018b) Preparation of the recycled and regenerated mesocarbon microbeads-based solid acid and its catalytic behaviors for hydrolysis of cellulose. Bioresour Technol 270:166–171
Li Y, Shen S, Wang C, Peng X, Yuan S (2018c) The effect of difference in chemical composition between cellulose and lignin on carbon based solid acids applied for cellulose hydrolysis. Cellulose 25:1851–1863
Lin X, Wu L, Huang S, Qin Y, Qiu X, Lou H (2019) Effect of lignin-based amphiphilic polymers on the cellulase adsorption and enzymatic hydrolysis kinetics of cellulose. Carbohydr Polym 207:52–58
Liu T, Li Z, Li W, Shi C, Wang Y (2013) Preparation and characterization of biomass carbon-based solid acid catalyst for the esterification of oleic acid with methanol. Bioresour Technol 133:618–621
Mika LT, Csefalvay E, Nemeth A (2017) Catalytic conversion of carbohydrates to initial platform chemicals: chemistry and sustainability. Chem Rev 118:505–613
Mission EG, Quitain AT, Sasaki M, Kida T (2017) Synergizing graphene oxide with microwave irradiation for efficient cellulose depolymerization into glucose. Green Chem 19:3831–3843
Nagalakshmi M, Karthikeyan C, Anusuya N, Brundha C, Basu MJ, Karuppuchamy S (2017) Synthesis of TiO2 nanofiber for photocatalytic and antibacterial applications. J Mater Sci Mater Electron 28:15915–15920
Ngaosuwan K, Goodwin JG, Prasertdham P (2016) A green sulfonated carbon-based catalyst derived from coffee residue for esterification. Renew Energy 86:262–269
Onda A, Ochi T, Yanagisawa K (2008) Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem 10:1033–1037
Ordomsky VV, Schouten JC, van der Schaaf J, Nijhuis TA (2012) Foam supported sulfonated polystyrene as a new acidic material for catalytic reactions. Chem Eng J 207:218–225
Pan H, Liu X, Zhang H, Yang K, Huang S, Yang S (2017) Multi-SO3H functionalized mesoporous polymeric acid catalyst for biodiesel production and fructose-to-biodiesel additive conversion. Renew Energy 107:245–252
Pang Q, Wang L, Yang H, Jia L, Pan X, Qiu C (2014) Cellulose-derived carbon bearing –Cl and –SO3H groups as a highly selective catalyst for the hydrolysis of cellulose to glucose. RSC Adv 4:41212–41218
Piqueras CM, Cabeza Á, Gallina G, Cantero DA, García-Serna J, Cocero MJ (2017) Online integrated fractionation-hydrolysis of lignocellulosic biomass using sub- and supercritical water. Chem Eng J 308:110–125
Promdej C, Matsumura Y (2011) Temperature effect on hydrothermal decomposition of glucose in sub- and supercritical water. Ind Eng Chem Res 50:8492–8497
Qi X, Watanabe M, Aida TM, Smith RL (2011) Catalytic conversion of cellulose into 5-hydroxymethylfurfural in high yields via a two-step process. Cellulose 18:1327–1333
Qin L, Liu L, Li WC, Zhu JQ, Li BZ, Yuan YJ (2016) Evaluation of soluble fraction and enzymatic residual fraction of dilute dry acid, ethylenediamine, and steam explosion pretreated corn stover on the enzymatic hydrolysis of cellulose. Bioresour Technol 209:172–179
Saeman JF (1945) Kinetics of wood saccharification-hydrolysis of cellulose and decomposition of sugars in dilute acid at high temperature. Ind Eng Chem 37:43–52
Sasaki M, Fang Z, Fukushima Y, Adschiri T, Arai K (2000) Dissolution and hydrolysis of cellulose in subcritical and supercritical water. Ind Eng Chem Res 39:2883–2890
Sasaki M, Adschiri T, Arai K (2004) Kinetics of cellulose conversion at 25 MPa in sub- and supercritical water. AIChE J 50:192–202
Shen J, Wyman CE (2012) Hydrochloric acid-catalyzed levulinic acid formation from cellulose: data and kinetic model to maximize yields. AIChE J 58:236–246
Shen F, Smith RL, Li L, Yan L, Qi X (2017) Eco-friendly method for efficient conversion of cellulose into levulinic acid in pure water with cellulase-mimetic solid acid catalyst. ACS Sustain Chem Eng 5:2421–2427
Shen F, Guo T, Bai C, Qiu M, Qi X (2018) Hydrolysis of cellulose with one-pot synthesized sulfonated carbonaceous solid acid. Fuel Process Technol 169:244–247
Shuai L, Pan X (2012) Hydrolysis of cellulose by cellulase-mimetic solid catalyst. Energy Environ Sci 5:6889–6894
Su TC, Fang Z, Zhang F, Luo J, Li XK (2015) Hydrolysis of selected tropical plant wastes catalyzed by a magnetic carbonaceous acid with microwave. Sci Rep 5:17538
Suganuma S, Nakajima K, Kitano M, Yamaguchi D, Kato H, Hayashi S, Hara M (2008) Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J Am Chem Soc 130:12787–12793
Sutarlie L, Yang KL (2013) Hybrid cellulase aggregate with a silica core for hydrolysis of cellulose and biomass. J Colloid Interface Sci 411:76–81
Tyufekchiev M, Duan P, Schmidt-Rohr K, Granados-Focil S, Timko T, Emmert MH (2018) Cellulase-inspired solid acids for cellulose hydrolysis structural explanations for high catalytic activity. ACS Catal 8:1464–1468
Wu B, Wang Z, Gong QM, Song HH, Liang J (2008) Fabrication and mechanical properties of in situ prepared mesocarbon microbead/carbon nanotube composites. Mater Sci Eng A 487:271–277
Wu M, Wang Y, Wang D, Tan M, Li P, Wu W, Tsubaki N (2016) SO3H-modified petroleum coke derived porous carbon as an efficient solid acid catalyst for esterification of oleic acid. J Porous Mater 23:263–271
Yang Q, Pan X (2016) Synthesis and application of bifunctional porous polymers bearing chloride and sulfonic acid as cellulase-mimetic solid acids for cellulose hydrolysis. BioEnergy Res 9:578–586
Yuan S, Li T, Wang Y, Cai B, Wen X, Shen S, Peng X, Li Y (2019) Double-adsorption functional carbon based solid acids derived from copyrolysis of PVC and PE for cellulose hydrolysis. Fuel 237:895–902
Zhang M, Zhou Q, Li A, Shuang C, Wang W, Wang M (2013) A magnetic sorbent for the efficient and rapid extraction of organic micropollutants from large-volume environmental water samples. J Chromatogr A 1316:44–52
Acknowledgments
The authors are very grateful for the financial support of the National Natural Science Foundation of China NSFC (No. 51174144), the Key Research and Development Program of Shanxi Province (No. 201703D12111437) and the Graduate Science and Technology Innovation Fund Project of Shanxi. (No. 2019BY059).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, HX., Zhang, X., Wang, Q. et al. Study on the hydrolysis of cellulose with the regenerable and recyclable multifunctional solid acid as a catalyst and its catalytic hydrolytic kinetics. Cellulose 27, 285–300 (2020). https://doi.org/10.1007/s10570-019-02777-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02777-3