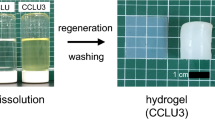
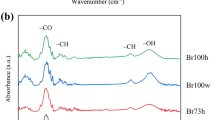
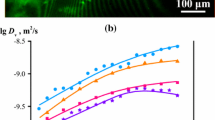
Abstract
Surface and structural properties of cellulose hydrogel prepared from LiOH/urea solvent with alcoholic coagulation were examined. As coagulants, alcohols from methanol to butanol were employed. Alcohol with high water miscibility (MeOH, EtOH, 1-PrOH, 2-PrOH, and t-BuOH) gave a nano-porous structure consisting of a fibrous network of cellulose, while alcohol with low water miscibility (1-BuOH, 2-BuOH, i-BuOH) showed aggregation of a fibrous structure because of large shrinkage during the coagulation process. Congo red adsorption measurement showed that an increase in the carbon number of alcohol brought about a less hydrophobic surface. This is likely to occur because the alkali/urea/cellulose complex was formed during the coagulation process in the case of ethanol, propanol, and butanol, leading to a higher crystalline content of cellulose II, the surface of which is thought to be highly hydrophilic.





Similar content being viewed by others
References
Cai J, Zhang L (2005) Rapid dissolution of cellulose in LiOH/urea and NaOH/urea aqueous solutions. Macromol Biosci 5:539–549
Cai J, Kimura S, Wada M, Kuga S, Zhang L (2008) Cellulose aerogels from aqueous alkali hydroxide–urea solution. ChemSusChem 1:149–154
Cross CF, Bevan BT, Beadle C (1893) Thiokohlensäureester der cellulose. Ber Dtsch Chem Ges 26:1090–1097
Engle RE, Purdie N, Hyatt JA (1994) Induced circular dichroism study of the aqueous solution complexation of cello-oligosaccharides and related polysaccharides with aromatic dyes. Carbohydr Res 265:181–195
Fink HP, Weigel P, Purz HJ, Ganster J (2001) Structure formation of regenerated cellulose materials from NMMO-solutions. Prog Polym Sci 26:1473–1524
Gavillon R, Budtova T (2008) Aerocellulose: new highly porous cellulose prepared from Cellulose-NaOH aqueous solutions. Biomacromolecules 9:269–277
Head-Gordon H, Hura G (2002) Water structure from scattering experiments and simulation. Chem Rev 102:2651–2670
Innerlohinger J, Weber HK, Kraft G (2006) Aerocellulose: aerogels and aerogel-like materials made from cellulose. Macromol Symp 244:126–135
Isobe N, Kim UJ, Kimura S, Wada M, Kuga S (2011a) Internal surface polarity of regenerated cellulose gel depends on the species used as coagulant. J Colloid Interface Sci 359:194–201
Isobe N, Sekine M, Kimura S, Wada M, Kuga S (2011b) Anomalous reinforcing effects in cellulose gel-based polymeric nanocomposites. Cellulose 18:327–333
Isobe N, Kimura S, Wada M, Kuga S (2012) Mechanism of cellulose gelation from aqueous alkali-urea solution. Carbohydr Polym 89:1298–1300
Jin H, Nishiyama Y, Wada M, Kuga S (2004) Nanofibrillar cellulose aerogels. Colloids and Surfaces A: physicochem. Eng Aspects 240:63–67
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393
Mazeau K, Wyszomirski M (2012) Modelling of Congo red adsorption on the hydrophobic surface of cellulose using molecular dynamics. Cellulose 19:1495–1506
Scweizer E (1857) Das kupferoxyd–ammoniak, ein auflösungsmittel für die pflanzenfaser. J Prakt Chem 72:109–111
Sescousse R, Gavillon R, Budtova T (2011) Aerocellulose from cellulose–ionic liquid solutions: preparation, properties and comparison with cellulose–NaOH and cellulose–NMMO routes. Carbohydr Polym 83:1766–1774
Vahvaselkä KS, Serimaa R, Torkkeli M (1995) Determination of liquid structures of the primary alcohols methanol, ethanol, 1-propanol, 1-butanol and 1-octanol by X-ray scattering. J Appl Cryst 28:189–195
van de Witte P, Dijkstra PJ, van den Berg JWA, Feijen J (1996) Phase separation process in polymer solutions in relation to membrane separation. J Membr Sci 117:1–31
Wang Z, Liu S, Matsumoto Y, Kuga S (2012) Cellulose gel and aerogel from LiCl/DMSO solution. Cellulose 19:393–399
Warwicker JO, Wright A (1967) Function of sheets of cellulose chains in swelling reactions on cellulose. J App Polym Sci 11:659–671
Yuguchi Y, Hirotsu T, Hosokawa J (2005) Structural characteristics of xyloglucan—Congo red aggregates as observed by small angle X-ray scattering. Cellulose 12:469–477
Acknowledgments
This study was partly supported by a Grant-in-Aid for Scientific Research (no. 23580226) and a Grant-in-Aid for JSPS fellows (no. 24–7759). N.I. acknowledges financial support from the JSPS Research Fellowship for Young Scientists. The authors thank Japan Synchrotron Research Institute (JASRI) for provision of beam time at BL40B2 in SPring-8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Isobe, N., Nishiyama, Y., Kimura, S. et al. Origin of hydrophilicity of cellulose hydrogel from aqueous LiOH/urea solvent coagulated with alkyl alcohols. Cellulose 21, 1043–1050 (2014). https://doi.org/10.1007/s10570-013-0080-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-0080-7