Abstract
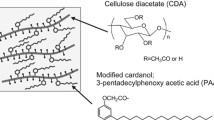
Novel cellulose-based bioplastics, mainly using inedible plant resources, were produced by bonding cellulose diacetate (CDA), a modified cardanol, and additional aliphatic and aromatic components. Cardanol is a phenol derivative with a linear unsaturated hydrocarbon side chain (carbon number: 15), derived from cashew nut shells. Esterification of the modified cardanol (3-pentadecylphenoxy acetic acid: PAA) and CDA resulted in a thermoplastic PAA-bonded CDA with high tenacity (long elongation while keeping maximum bending strength), heat resistance, and water resistance. These properties were better than those of a conventional CDA composite consisting of CDA and a conventional plasticizer. By comparing the PAA-bonded CDA with a CDA bonded with stearic acid (SA), which has a linear structure similar to that of PAA’s side chain but has no phenyl part, it was suggested that the linear side chain in PAA has a main role in these prominent properties of the PAA-bonded CDA, while the phenyl part in PAA has pronounced effects on its maximum bending strength and water resistance. Additional bonding of linear alkanoic acids, especially SA as aliphatic components improved the PAA-bonded CDA’s impact strength, and additional bonding of benzoic acid (BA) as an aromatic component further increased its maximum bending strength and elastic modulus. These components improved the thermoplasticity and water resistance of the PAA-bonded CDA while maintaining its high heat resistance relatively well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the face of petroleum resource depletion and global warming, biomass-based plastics (bioplastics), made using renewable plant resources as raw materials, have been attracting increased attention in recent years for use in environmentally conscious applications. At present, mass-produced bioplastics such as polylactic acid (PLA) (Li et al. 2010; Serizawa et al. 2006) use starch, which is generally produced from edible plants, as the main resource. Fears of future food shortages, however, are driving an effort to use plant resources that are not edible.
We have produced advanced bioplastics to be used in durable products by mainly using two readily procurable inedible plant resources: cellulose, the main component of most plants and the most abundant non-food plant resource produced in the ground, and cardanol, a uniquely structured phenol derivative with a liner alkyl side chain, derived from cashew nut shells generated in large amounts as a byproduct.
Cellulose is a polysaccharide consisting of d-glucose units linked together by β1-4 glycosidic bonds into linear chains. It has an extensive hydrogen-bonded and partially crystallized structure that makes it very stiff, non-melt processable, and insoluble in water and most organic solvents. Esterification is commonly used to produce thermoplastics from cellulose, mainly by using various acids such as acetic acid, propionic acid, and nitric acid (Zepnik et al. 2010). Because of these cellulose derivatives’ narrow windows between the melting and degradation temperatures, large amounts of external plasticizers such as glycerin, polyethylene glycol, and triethyl citrate (TEC) generally must be added (Hwan-Man et al. 2004). However, adding the external plasticizers can reduce the bending strength and heat resistance of cellulose ester composites.
To overcome this problem, a number of studies have been made on modifying cellulose and its derivatives by bonding them with long chain alkyl compounds or by grafting various polymers with them. The bonding and grafting agents used serve as internal plasticizers and provide additional properties. Several studies investigated the use of cellulose acetates, which are soluble in several common organic solvents, as a precursor in the grafting of various linear polymers (Roy et al. 2009), including ε-caprolactone-l-lactide copolymers (Nie and Narayan 1994), PLA (Yoshioka et al. 1999; Teramoto and Nishio 2003), and styrene-maleic anhydride random copolymers (Teramoto and Nishio 2004). More recent studies have investigated the use of pure cellulose as a precursor by using special solvents such as N,N-dimethylacetamide/lithium chloride and ion liquids such as 1-allyl-3-methylimidazolium chloride (Edgar et al. 1998) to dissolve cellulose. In these solvents, long chain alkanoic acids (carbon number: 12–20) were bonded (Sealey et al. 1996), and also graft copolymerization was conducted using various monomers such as lactic acid (Chenghu et al. 2009) and methacrylate (Enomoto-Rogers et al. 2009). However, the cellulose-based bioplastics produced in these studies did not have sufficient mechanical strength, heat resistance, and water resistance for practical use in various durable products. Moreover, the bonding and grafting agents used were made from a petroleum product or starch.
Cardanol is a principal organic ingredient (about 30 wt%) in cashew nut shells (Kumar et al. 2002; Lubi and Thachil 2000). Cashew nuts are a renewable agricultural resource, and cashew trees (Anacardium occidentale L.) are widely planted in tropical countries such as India and Vietnam. In processing cashew nuts, large quantities of shells are generated as an inedible byproduct. Cardanol is a phenol derivative consisting of a linear unsaturated hydrocarbon as a flexible and hydrophobic long side chain (carbon number: 15) and phenol as a rigid aromatic part with a reactive hydroxide group. It is superior to common phenol derivatives in terms of many practical characteristics such as water resistance, flexibility, and friction resistance when used as a major component in thermosetting plastics such as phenol resins and epoxy resins (Devi and Srivastava 2007; Antony and Pillai 1993). These resins are mainly used in surface coatings, insulating materials, adhesives, and friction materials.
However, there have been few studies on cellulose derivatives bonded with cardanol. While cardanol has been bonded to natural cellulose fiber (in sheet form) to retard water penetration (Maffezzoli et al. 2004), to the best of the authors’ knowledge, there have been no reports of thermoplastics produced by bonding cardanol with cellulose derivatives and their durable properties such as mechanical strength and heat resistance.
Very recently, we investigated the hydrophobic, mechanical, and thermal characteristics of a thermoplastic cellulose diacetate (CDA) bonded with a modified cardanol (3-pentadecylphenoxy acetic acid: PAA) and reported them briefly in a note (Iji et al. 2011). Cardanol was modified to enhance its reactivity with CDA. The unsaturated bonds in its alkyl side chain were changed to saturated ones by adding hydrogen and the hydroxide group of its phenol part was changed to acetic acid chloride group. Esterification of the resulting PAA chloride with CDA resulted in a thermoplastic PAA-bonded CDA with high tenacity (long elongation while keeping maximum bending strength), heat resistance, and water resistance. All of these properties were considerably better than those of a conventional CDA composite consisting of CDA and a conventional external plasticizer (TEC). These prominent properties came from the combination of cardanol, which is flexible and hydrophobic, and CDA, which is rigid and resistant to deformation. However, the structural effect of the modified cardanol (PAA) moiety on the characteristics of the PAA-bonded CDA was insufficiently investigated. Moreover, merely adjusting of the bonding ratio of PAA had a limited effect on further improving these characteristics as well as improving the impact strength of the resulting resins.
In the study reported here, we first estimated the PAA-bonded CDA’s mechanical characteristics and other practical properties, including thermal resistance, thermoplasticity and water resistance, compared with those of CDA composites consisting of CDA and the external plasticizer (TEC). Next, we investigated the structural features of PAA linked with the CDA molecules, in particular, the manner in which its linear side chain and its phenyl part affect the PAA-bonded CDA’s characteristics, by comparing the PAA-bonded CDA with the CDA bonded with stearic acid (SA: C17H35COOH), which has a linear structure similar to that of PAA’s side chain but has no phenyl part. Furthermore, to improve these properties of the PAA-bonded CDA, we additionally bond it with aliphatic and aromatic components. We estimated three kinds of linear alkyanoic acids with various lengths, heptanoic acid (HA: C6H13COOH), lauric acid (LA: C11H23COOH), and SA as the aliphatic components, and benzoic acid (BA: C6H5COOH) as the aromatic component. Finally, we compared the representative PAA-bonded CDA with additional components with reference current plastics including the CDA composite with plasticizer (TEC), the representative mass-produced bioplastic: PLA, a bioplastic derived from cater beans: Polyamide 11 (PA11), and a petroleum-based plastic used in durable products: Acrylonitrile-butadiene-styrene (ABS) resin.
Experimental
Chemicals
CDA (LM-80, DS* of acetic acid: 2.1, viscosity: 200 mPa ·s/6 wt% in acetone) was supplied by Daicel Chemical Industries, Ltd. (Japan) (*degree of substitution: ratio of replacing three hydroxide groups in one glucose unit in cellulose with acetic acids). Hydrogenated cardanol (m–n-pentadecyl phenol), in which the unsaturated bonds in the alkyl side chain were changed to saturated ones by adding hydrogen, was supplied by ACROS Organics Co.(USA). The change ratio was more than 99 %, as measured by the company. Chloroacetic acid, chloroform, acetone, 1,4-dioxane, methanol, hexane, diethyl ether, triethyl amine, sodium hydroxide, hydrochloric acid, oxalyl chloride, and N,N-dimethylformamide were the analytical reagents and were used as received from Kanto Chemical Co., Inc. (Japan) without further purification. The plasticizer: TEC was supplied by Pfizer Inc. (USA). PLA(TE-4000) was supplied by Unitika Ltd. (Japan). PA11: poly 11-aminoundecanoic acid (Rilson BMFO) was supplied by Arkema Japan Ltd. (Japan). ABS resin (GA-701) was supplied by Nippon A&L Co. (Japan). As additional components, SA chloride was received from Kanto Chemical Co., Inc. (Japan), and BA chloride, HA chloride, and LA chloride were received from Tokyo Chemistry Industry Co. Ltd. (Japan). These additional components were used without further purification.
Preparation of cellulose ester derivatives
The preparation process of the PAA-bonded CDA with additional components is shown in Fig. 1.
We prepared PAA chloride as follows. The hydrogenated cardanol (300 g, 0.99 mol) and sodium hydroxide (90 g, 2.25 mol) were dissolved in a mixture of water (150 ml) and methanol (300 ml). Chloroacetic acid (99 g, 1.05 mol) in methanol (110 ml) was slowly added to the mixture, and then it was refluxed for 6 h. After the reaction, the product was acidified with hydrochloric acid (37 %, 120 g). The resulting material was extracted with diethyl ether and then isolated from the solvent by using a rotary evaporator. The product was further purified by soxhlet extraction with hexane, resulting in 210 g (yield 60 %) of the product (PAA), as determined using a hydrogen-nuclear magnetic resonance (1H-NMR) spectrometer (BRUKER AVANCE 400; 400-MHz operation, Bruker Co., USA). (1H-NMR [DMSO-d6, ppm]: δ0.86 (3H, R–CH3), 1.26 (24H, –(CH2)n–), 1.6, 2.5 (2H, –CH2–Ar), 4.60 (2H, –CH2–CO), 6.65–6.73 (1H, Ar–H), 6.75 (1H, Ar–H), 7.15 (1H, Ar–H), 12.95 (1H, –COOH)). Slow addition of oxalyl chloride (20 ml, 0.23 mol) to the solution of the resulting product (56 g, 0.15 mol) in dehydrated chloroform (450 ml) with a few drops (0.4 ml) of N,N-dimethylformamide (DMF) as a catalyst under stirring at room temperature for 15 h produced the desired PAA chloride with a yield of about 100 %. The chemical structure of the product was identified using an 1H-NMR spectrometer: peak data [1H-NMR [DMSO-d6, ppm]: δ0.86 (3H, R–CH3), 1.26 (24H, –(CH2)n–), 1.6, 2.5 (2H, –CH2–Ar), 4.60 (2H, –CH2–CO), 6.65–6.73 (1H, Ar–H), 6.75 (1H, Ar–H), 7.15 (1H, Ar–H)].
The PAA chloride was bonded with CDA as follows. CDA (10.0 g) was dissolved in dehydrated 1,4-dioxane (200 ml) by stirring at 90 °C under a dry nitrogen atmosphere. After it was cooled to room temperature, 5.0 ml (0.036 mol) of triethyl amine as an acid scavenger was added. A solution of PAA chloride (6.9 g, 0.018 mol) in dehydrated 1, 4-dioxane (100 ml) was then slowly added dropwise, and the mixture was refluxed for 5 h under a nitrogen atmosphere to promote grafting reaction. The reaction solution was poured into 2 L of methanol being vigorously stirred to isolate a solid product. The product was filtered by suction filtration and washed three times with 1 L of methanol. The resultant product was dried overnight at room temperature and then dried under vacuum for 5 h at 105 °C to obtain the target PAA-bonded CDA (yield about 96 %). The final product (PAA-bonded CDA) was determined using the 1H-NMR spectrometer. The DS of PAA was calculated by means of the ratio between the area corresponding to the proton resonance of the methyl protons of the acetate group (δ1.80–2.20) and the corresponding resonance for the methyl protons of CAA (δ0.86). As a result, the DS of PAA was 0.33 (the ratio of bonded PAA content calculated by using it was 31 wt%). The molecular weight of the PAA-bonded CDA was estimated by gel permeation chromatography (GPC: Shimadzu LC-VP system with a Shim-pack GPC-8025C and GPC-80MC column, Shimadzu Co., Japan), calibrated using polystyrene standards (solvent: chloroform). The number average molecular weight (Mn) of the PAA-bonded CDA was about 76,000.
The method described above was used to prepare other PAA-bonded CDA samples, using other bonding ratios of PAA by changing only the amounts of the PAA and triethyl amine. The method was also used to prepare SA-bonded CDA samples by using SA chloride and CDA.
Additional aliphatic and aromatic components were bonded with the PAA-bonded CDA (DS of PAA: 0.33) as follows. The aliphatic and aromatic acid chlorides (BA chloride, HA chloride, LA chloride SA chloride, and PAA chloride) were added to CDA in amounts three to four times greater than the amounts we aimed to bond. Triethyl amine was added in an equivalent weight 1.5 times greater than that of these additional components added. The reaction condition and the resultant products’ recovery method were as mentioned above.
In particular, the resulting PAA-bonded CDA with SA, and PAA-bonded CDA with the mixture of SA and BA were further purified because considerable amounts of unreacting components remained in the resultant products. Therefore, after dissolving the resultants (20 g) in chloroform (2L), methanol of 4L was poured into the solutions being vigorously stirred to isolate solid products. The amounts of the additional components in the products were determined using the 1H-NMR spectrometer as mentioned above.
The reference CDA composite containing CDA and the plasticizer (TEC) was prepared by mixing them with an extruder (HAAKE MiniLab Rheomex extruder: Model CTW5, Thermo Fisher Scientific Co., Germany) at 200 °C.
Measurements
For the mechanical tests, the PAA-bonded CDA and its derivatives with additional components, the CDA composite containing the plasticizer (TEC), PLA, PA11 and ABS resin were molded into the test pieces by using an injection-molding machine (HAAKE Mini Jet II, Thermo Fisher Scientific Co., Germany) operating at 210 °C. The PLA test pieces were annealed at 100 °C for 4 h. Because CDA alone was hardly melted, its test pieces were molded by dissolving CDA in acetone, casting the solution in a metal mold and volatizing acetone at room temperature. However, the thick pieces of CDA for measuring its mechanical characteristics could not be formed by the method, and therefore these characteristics were not evaluated. The mechanical characteristics of the molded samples were measured by bending testing in accordance with ASTM D790 by using a bending testing instrument (INSTRON 5567, Instron Co., USA). The test pieces of the molded resin samples in the bending test were 2.4 mm thick, 80 mm long, and 12.4 mm wide. Furthermore, Izod-impact testing was conducted in accordance with JISK7110 using an impact testing instrument (Universal Impact Tester C1, Toyo Seiki CO., Japan). The test pieces with notches were 2.4 mm thick, 80 mm long, and 12.4 mm wide.
To measure the glass transition temperature (Tg) of the resulting resin samples, differential scanning calorimetry (DSC) analysis of the samples was conducted using a differential scanning calorimeter (DSC 6200/EXSTAR6000, Seiko Instrument Inc., Japan). The same thermal history as before the measurement was performed by heating each sample from −100 to 230 °C at a scanning rate of 10 °C per minute, then keeping it at 230 °C for 3 min, and finally quenching it to room temperature.
The melt flow rate (MFR), which is the weight of a melted sample passing through the capillary (size: 10 mm × 2 mmφ) in 10 min, was estimated by using a capillary rheometer (CFT-500D, Shimadzu Co., Japan) at 200 °C with a 500 kgf/cm2 load. Each sample was kept for 5 h in a 105 °C preheated oven before the MFR test to remove humidity.
The water resistance was evaluated by calculating the water absorption ratios of the molded samples of the bending test by weighing and measuring them before and after soaking them for 24 h in distilled water at room temperature.
Results and discussion
Characteristics of modified cardanol-bonded cellulose diacetate
We evaluated the characteristics of CDA bonded with the modified cardanol (PAA- bonded CDA): its mechanical properties (maximum bending strength, elastic modulus, elongation, impact strength), heat resistance (glass transition temperature: Tg), thermoplasticity (melt flow rate: MFR), and water resistance (water absorption ratio), which are required properties when the resulting resins are used in durable products. We also compared the PAA-bonded CDA with CDA alone and CDA composites with the external plasticizer (TEC).
The hypothesized structure of the PAA-bonded CDA is illustrated in Fig. 2a. Table 1 lists the measurements results. The PAA-bonded CDA showed good thermoplasticity without external plasticizers, high tenacity (long elongation while keeping maximum bending strength), heat resistance, and water resistance. These properties were better than those of the CDA composites consisting of CDA and the external plasticizer. Its water resistance was considerably higher than that of CDA alone.
The good thermoplasticity and the elongation are due to the PAA moiety linked with the CDA molecules, which functions as an inner plasticizer, enabling the CDA molecules to slip during deformation of the PAA-bonded CDA in these measurements. The maximum bending strength was maintained, mainly due to the chemical and physical interactions among the PAA moieties linked with the CDA molecules. These interactions resulted from the high affinity and entanglement through the long alkyl side chain of PAA. In addition, as will be noted in more detail later, the rigid phenyl part in PAA restricted the motion of the PAA-bonded CDA molecules, which helps maintain the maximum bending strength and elastic modulus.
On the other hand, the considerable decrease in the maximum bending strength of the CDA composites with the external plasticizer was caused by low interaction between CDA and the plasticizer, which was simply mixed with the CDA. However, the plasticizer functions to increase the composites’ impact strength, because it reduces impact energy by enabling the CDA molecules to slip when the impact is added.
Although Tg of the PAA-bonded CDA decreased as the proportion of PAA was increased, it remained higher than that of the CDA composites with the external plasticizer. Since Tg is mainly affected by the ease of movement of polymer molecules during heating, the maintaining of the high Tg by the PAA-bonded CDA was apparently due to high interaction among the long alkyl side chains of the PAA moiety linked with the CDA molecules. The lower Tg of the CDA composites containing the external plasticizer resulted from the low interaction between CDA and the plasticizer.
The high water resistance (low water absorption) of the PAA-bonded CDA was mainly due to replacement of the hydrophilic hydroxide groups in the CDA with the hydrophobic PAA moiety. Moreover, the flexible long alkyl side chain of the PAA moiety can protect the remaining hydroxide groups in the PAA-bonded CDA from water absorption.
Comparison of modified cardanol-bonded cellulose diacetate and stearic acid-bonded cellulose diacetate
We investigated the structural features of the PAA-bonded CDA, in particular, the effects of PAA’s linear side chain and phenyl part on the mechanical and other practical properties of the PAA-bonded CDA. For the purpose, we compared these properties with those of SA-bonded CDA; SA has a linear structure similar to that of PAA’s side chain but has no phenyl part. Table 1 shows these results.
The maximum bending strength and elastic modulus of the PAA-bonded CDA were higher than those of the SA-bonded CDA (Fig. 3). This reveals that the rigid phenyl part of PAA restricts the movement of the CDA molecules during the bending measurement, resulting in the prominent properties. On the other hand, in terms of the impact strength, the PAA-bonded CDA was inferior to the SA-bonded CDA.
The impact strength of plastics is enhanced by the inclusion of fine flexible domains as shock absorbers, which are mainly fine elastomeric particles (Li and Turng 2006). The long linear alkyl chain in SA can form such flexible domains. In DSC measurements (Fig. 4a), the SA-bonded CDA showed a strong secondary dispersion peak at a lower temperature (−20 °C) than the Tg (145 °C), suggesting the formation of flexible domains derived from SA to improve the impact strength. On the other hand, in the DSC measurements of the PAA-bonded CDA (Fig. 4b), we observed a very small secondary dispersion peak at a low temperature. This showed that PAA’s phenyl part, which hardly deformed, retards the formation of the flexible domains by its long linear side chain, thus reducing the PAA-bonded CDA’s impact strength.
On the other hand, the PAA-bonded CDA’s Tg was almost the same as that of the SA-bonded CDA. The former’s water resistance was increased while its thermoplasticity was decreased, owing to PAA’s phenyl part, which is hydrophobic and not easily deformed.
These results suggested the roles of the long linear side chain and the phenyl part of the PAA moiety linked with CDA molecules; the linear side chain mainly produces the prominent properties of the PAA-bonded CDA (thermoplasticity, tenacity, heat resistance and water resistance), while the phenyl part functions to increase the maximum bending strength, elastic modulus and water resistance but adversely affects the impact strength and thermoplasticity.
Therefore, we consider that adequate additional bonding of flexible linear components with the PAA-bonded CDA can improve its impact strength, while mostly maintaining other characteristics including thermoplasticity, heat resistance and water resistance. We also consider that additional bonding of rigid aromatic components can improve its maximum bending strength and elastic modulus.
Additional bonding of liner aliphatic and aromatic components with modified cardanol-bonded cellulose diacetate
The hypothesized structure of the PAA-bonded CDA with additional aliphatic and aromatic components is illustrated in Fig. 2b. The chlorides of the linear alkanoic acids with different lengths as aliphatic components, BA as an aromatic component, the mixtures of SA and BA, and PAA as the were linked to the PAA-bonded CDA (DS: PAA of 0.33, acetic acid of 2.1). We evaluated the resulting PAA-bonded with the additional components and compared their representatives with the current reference plastics including the CDA composite with the external plasticizer (TEC, 29 wt%), PLA, PA11, and ABS resin. All the results are shown in Table 2.
In the bending test as shown in Fig. 5, additional bonding of BA with the PAA-bonded CDA increased its elastic modulus while maintaining the maximum bending strength, but the elongation was decreased, when the proportion of BA was increased. This result is due to the rigidity of the phenyl part in BA. On the contrary, additional bonding of the linear alkanoic acids and PAA as the reference decreased the elastic modulus and the maximum strength of the PAA-bonded CDA, as the proportions of these additional components and their lengths were increased. These results are owing to the increase of the flexible part volumes derived from these long linear alkyl units in these components. Additional bonding of the mixtures of BA and SA showed almost the same result as bonding of SA alone. This reveals that the bonding of BA together with SA insufficiently produces the elastic modulus and the maximum bending strength, due to the priority effect of SA.
As shown in Fig. 6, the PAA-bonded CDA’s impact strength was increased by the additional bonding of the linear alkanoic acids, in particular, SA, at the relatively high proportions. We hypothesize that additionally bonded long linear alkanoic acids such as SA can form such flexible domains as shock absorbers, as indicated above. On the other hand, additional bonding of BA, PAA, and the mixture of BA and SA decreased it at the relatively high proportions. It is suggested that the rigid phenyl parts in BA and PAA do not form such flexible domains and retards their formation produced by the long linear parts in PAA and SA.
Figure 7 shows Tg of the PAA-bonded CDAs with the additional components. Although Tg was decreased as the proportion of these additional components was increased, additional bonding of SA, BA, and their mixtures maintained high Tg relatively well; SA in particular maintained it sufficiently. Since Tg is in general mainly affected by the ease in which polymer molecules move during heating, we can conclude that the maintaining of the high Tg by additional bonding of SA was mainly due to the high interaction (high affinity and entanglement) between the SA moieties linked with the PAA-bonded CDA molecules. On the other hand, additionally bonded BA retards the motion of the PAA-bonded CDA molecules during heating due to the rigidity of its phenyl part, resulting in the high Tg. Additional bonding of the mixtures of BA and SA produces the Tg as a result of both of these effects.
As shown in Fig. 8, the PAA-bonded CDAs with the linear alkanoic acids and PAA as additional components showed the increases in MFR as the proportion of these components was increased. This means that these linear components function as effective internal plasticizers for the PAA-bonded CDA by reducing the interactions among the PAA-bonded CDA molecules. Although there is no significant difference in MFR between the PAA-bonded CDAs with these linear components with different lengths, they showed different Tg values, as mentioned above. The MFR measurements were conducted at a higher temperature (200 °C, similar to the molding condition) than the Tg of the PAA-bonded CDAs with the linear components, resulting in enhanced activation of the molecules’ motion. This causes the motion of the molecules to surpass their interactions, which considerably affect their Tg.
On the other hand, compared with additional bonding of these linear components, additional bonding of BA was insufficient to increase the MFR of the PAA-bonded CDA. This showed that the BA component functions less as an internal plasticizer than the linear components because the rigid phenyl part of BA becomes something of an obstacle to the slippage among the PAA-bonded CDA molecules during heating.
Figure 9 shows the water resistance (water absorption ratio). Bonding of all the additional components further improved the water resistance. The water absorption ratios were decreased substantially as the proportions of these additional components and the amount of the reference PAA were increased. The water inserted into them mainly attaches to the hydrophilic hydroxide groups of CDA, and therefore the degree of the substitution of these hydrophobic additional components for the hydroxide groups mostly affected the water absorption ratios. However, there was only a slight difference in the water absorption ratios between the PAA-bonded CDAs with these additional components at the same DS. Whereas the long linear components such as SA and PAA and the aromatic component such as BA have higher hydrophobicity than the other components, they insufficiently prevent the water insert. This is mainly because the additional components are partially located in the PAA-bonded CDA’s molecule structure as shown in Fig. 2.
As shown in Table 2, we compared the representative PAA-bonded CDAs with additional components with the CDA composites with the external plasticizer, PLA, PA11, and ABS resin as current reference plastics. We selected the PAA-bonded CDAs with LA (DS: 0.36), SA (DS: 0.38), and the mixture of BA and SA (DS: 0.11 of BA, 0.28 of SA) as the additional components (these data are italics) because they have good thermoplasticity (MFR: more than 1,000 g/min) comparable to that of the reference plastics and their other properties are higher than those of the other PAA-bonded CDAs with additional components (Fig. 8).
As a result, these representative PAA-bonded CDAs with the additional components showed higher heat resistance (Tg) than that of the reference plastics. Their water resistance was relatively near that of PLA, PA11, and ABS resin, and superior to that of the CDA composites with the plasticizer. Their maximum bending strength was lower than that of the reference plastics, but maintained to be near that of the CDA composite with plasticizer and PA11. Their elongation was sufficiently long, like that of the CDA composite with the plasticizer, PA11, and ABS resin, and considerably longer than that of PLA. Moreover, their impact strength, especially that of the PAA-bonded with SA, was higher than that of PLA and PA11 and near that of the CDA composite with the plasticizer, but lower than that of ABS resin.
Conclusions
We produced novel cellulose-based bioplastics by bonding cellulose diacetae (CDA), the modified cardanol derived from cashew nut shells, and additional aromatic and aliphatic components. Esterification of a modified cardanol (PAA) and CDA resulted in a thermoplastic PAA-bonded CDA with high tenacity, heat resistance, and water resistance. Comparing it with SA-bonded CDA revealed that the linear side chain in PAA mainly functions these prominent properties of the PAA-bonded CDA and the phenyl part in PAA has the effect of increasing the maximum bending strength, elastic modulus and water resistance. Additional bonding of aliphatic components, especially SA, with the PAA-bonded CDA increased its impact strength, and additional bonding of benzoic acid (BA) as an aromatic component further increased its maximum bending strength and elastic modulus. These components also improved the thermoplasticity and water resistance of the PAA-bonded CDA while maintaining its high heat resistance relatively well. Compared with reference current plastics including the CDA composite with plasticizer, PLA, PA11, and ABS resin, the selected PAA-bonded CDA with the additional components such as LA, SA, and the mixture of BA and SA at specific DS degrees were found to have higher heat resistance. Furthermore, their other practical properties were comparable to or near those of the reference plastics. We therefore conclude that the PAA-bonded CDAs with these additional components are thus promising cellulose-based bioplastics for use in various durable products requiring high thermal resistance and other practical properties.
Abbreviations
- DS:
-
Degree of substitution
- CDA:
-
Cellulose diacetate
- PAA:
-
3-pentadecylphenoxy acetic acid
- HA:
-
Heptanoic acid
- LA:
-
Lauric acid
- SA:
-
Stearic acid
- BA:
-
Benzoic acid
- TEC:
-
Triethyl citrate
- PLA:
-
Polylactic acid
- PA11:
-
Polyamide 11: poly(11-aminoundecanoic acid)
- ABS:
-
Acrylonitrile–butadiene–styrene
- Tg:
-
Glass transition temperature
- MFR:
-
Melt flow rate
- DSC:
-
Differential scanning calorimetry
- GPC:
-
Gel permeation chromatography
References
Antony R, Pillai CKS (1993) Synthesis and thermal characterization of chemically modified cardanol polymers. J Appl Polym Sci 49:2129–2135
Chenghu T, Jinming Z, Yuxia L, Jian Y, Jin W, Jun Z, Jiasong H (2009) Thermoplastic cellulose-graft-poly(L-lactide)copolymers homogeneously synthesized in an ionic liquid with 4-dimethylaminopyridine catalyst. Biomacromolecules 10(8):2013–2018
Devi A, Srivastava D (2007) Studies on blends of cardanol-based epoxidized novolac type phenolic resin and carboxyl-terminated polybutadiene (CTPB) I. Mater Sci Eng, A 458:336–347
Edgar KJ, Pecorini TJ, Glasser WG (1998) Preparation, properties, and perspective, cellulose derivatives, chapter 3: 38–60, ACS symposium series, vol 688. American Chemical Society, Washington, DC
Enomoto-Rogers Y, Kamitakahara H, Takano T, Nakatsubo F (2009) Cellulosic graft copolymer: poly(methacrylate) with cellulose side chain. Biomacromolecules 10:2110–2117
Hwan-Man P, Manjusri M, Lawrence TD, Mohanty AK (2004) Green nanocomposites from cellulose acetate Bioplastic and clay: effect of eco-friendly triethyl citrate plasticizer. Biomacromolecules 5:2281–2288
Iji M, Moon S, Tanaka S (2011) Hydrophobic, mechanical, and thermal characteristics of thermoplastic cellulose diacetate bonded with cardanol from cashew nut shell. Polym J 43:738–741
Kumar PP, Parmashivappa R, Vithayathil PJ, Rao PVS, Rao AS (2002) Process for isolation of cardanol from technical cashew (Anacardium occidentale L.) nut shell liquid. J Agric Food Chem 50:4705–4708
Li T, Turng L (2006) Polylactide, nanoclay, and core-shell rubber. Polym Eng Sci 1419–1427
Li S, Ernst W, Martin P (2010) Present and future development in plastics from biomass. Biofuels, Bioprod Biorefin 4(1):25–40
Lubi MC, Thachil ET (2000) Cashew Nut shell liquid (CNSL)- A verstile monomer for polymer synthesis. Des Monom Polym 3(2):123–153
Maffezzoli A, Calo E, Zurlo S, Giuseppe M, Tazia A, Stifani C (2004) Cardanol based matrix biocomposites reinforced with natural fibers. Com Sci Tech 64:839–845
Nie L, Narayan R (1994) Grafting cellulose acetate with styrene maleic anhydride random copolymers for improving dimensional stability. J Appl Polym Sci 54:601–617
Roy D, Semsarilar M, Guthrie JT, Perrier S (2009) Cellulose modification by polymer grafting: review. Chem Soc Rev 38:2046–2064
Sealey JE, Samaranayake G, Todd JG, Glasser WG (1996) Novel cellulose derivatives. IV. Preparation and thermal analysis of waxy esters of cellulose. J Polym Sci Part B Poly Phys 34:1613–1620
Serizawa S, Inoue K, Iji M (2006) Kenaf-fiber-reinforced poly(lactic acid) used for electronic products. J Appl Polym Sci 100(1):618–624
Teramoto Y, Nishio Y (2003) Cellulose diacetate-graft-poly(lactic acid)s: synthesis of wide-ranging compositions and their thermal and mechanical properties. Polymer 44:2701–2709
Teramoto Y, Nishio Y (2004) Biodegrable cellulose diacetate-graft-poly(L-lactide): enzymatic hydrolysis behavior and surface morphological characterization. Biomacromolecules 5:407–414
Yoshioka M, Hasegawa N, Shiraishi N (1999) Themoplastization of cellulose acetates by grafting of cyclic esters. Cellulose 6:193–212
Zepnik S, Kesselring A, Kopitzky R, Michels C (2010) Basics of cellulosics. Bioplastic Mag 5:44–47
Acknowledgments
The authors are grateful to Ms. Ai Meguro of Staff Service Engineering Co. and Mr. Hideki Honzawa of Tohoku Chemical Industries Ltd., for their supporting in the experiments we conducted. The work was supported by the Japan science and technology’s advanced low carbon technology research and development program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 2.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (http://creativecommons.org/licenses/by-nc-nd/2.0/)
About this article
Cite this article
Iji, M., Toyama, K. & Tanaka, S. Mechanical and other characteristics of cellulose ester bonded with modified cardanol from cashew nut shells and additional aliphatic and aromatic components. Cellulose 20, 559–569 (2013). https://doi.org/10.1007/s10570-012-9832-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-012-9832-z