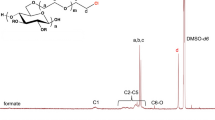
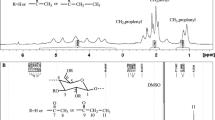
Abstract
The syntheses of several perdeuterated substances—some of them isotopically labeled (15N) in addition—are described, which act as direct solvents of cellulose either on their own, such as N-methylmorpholine N-oxide (NMMO), 1-butyl-3-methylimidazolium acetate (BMIM-OAc), or 1-ethyl-3-methylimidazolium acetate (EMIM-OAc), or in combination with auxiliaries, such as N,N-dimethylacetamide (DMAc—for the cellulose solvent DMAc/LiCl). NMMO-d11 (9) was obtained in an eight-step approach from non-labeled diglycolic acid (1) via diethylene glycol-d8 (4) and its bis-tosylate (5), which underwent ring closure with benzylamine to N-benzylmorpholine-d8 (6). Debenzylation, methylation and oxidation completed the synthetic sequence, which was also able to provide the 15N-labeled product (9a) by usage of 15N-benzylamine. DMAc-d9 (14) was obtained from deuterated acetic acid (10) and dimethylamine-d6–carbon dioxide complex (13) in a solvent-free gas-solid reaction with acidic alumina as the catalyst. Employing the CO2-complex of 15N-dimethylamine-d6 afforded the 15N-labeled product 15N-DMAc-d9 (14a) in a similar way. The two ionic liquids EMIM-OAc (21) and BMIM-OAc (22) were obtained from imidazole in three-step sequences starting with butylation and ethylation, respectively. The resulting 1-alkyl imidazoles were purified, and subsequently methylated according to a novel protocol using dimethylcarbonate-d6. Addition of acetic acid-d4 caused traceless degradation of the methylcarbonate anions and exchange for acetate. In all syntheses, the reaction steps were optimized with non-labeled compounds towards high yields and high reproducibility before entering the “hot runs” with deuterated and otherwise isotopically labeled material.



Similar content being viewed by others
References
Adelwöhrer C, Yoneda Y, Nakatsubo F, Rosenau T (2008) Synthesis of the perdeuterated cellulose solvents N-methylmorpholine N-oxide (NMMO-d11) and N,N-dimethylacetamide (DMAc-d9). J Labelled Comp Radiopharm 51:28–32. doi:10.1002/jlcr.1467
Barthel S, Heinze T (2006) Acylation and carbanilation of cellulose in ionic liquids. Green Chem 8:301. doi:10.1039/b513157j
Buchanan GW, Driega AB, Moghimi A, Bensimon C, Bourque K (1993) cis-Cyclohexano-9-crown-3 ether solid state and low-temperature solution stereochemistry as determined by X-ray crystallography and nuclear magnetic resonance spectroscopy. Can J Chem 71:951–958. doi:10.1139/v93-127
Chanzy H (1980) Quellung und Lösen von Cellulose im Aminoxid-Wasser-System. J Polym Sci Polym Phys Ed 18:1137–1144. doi:10.1002/pol.1980.180180517
Chanzy H, Nawrot S, Peguy A, Smith P (1982) The phase behavior of the quasiternary system N-methylmorpholine-N-oxide, water and cellulose. J Polym Sci 20:1909–1924
Dawsey TR, McCormick CL (1990) The lithium chloride/dimethylacetamide solvent for cellulose: a literature review. Macromol Chem Phys C30:405–440
Farbenind IG (1933) Patent DE 619754
Fedorova TA, Reshetov VA, Yakushkin MI, Velichko LS, Kotov VI (1982) Kinetics of synthesis of dimethylacetamides over aluminum oxide. J Appl Chem USSR (Engl Transl) 55:1968–1970. Zh Prikl Khim Leningrad 55:2140–2142
Heinze T, Schwikal K, Barthel S (2005) Ionic liquids as reaction medium in cellulose functionalization. Macromol Biosci 5:520. doi:10.1002/mabi.200500039
Hermanutz F, Meister F, Uerdingen E (2006) New developments in the manufacture of cellulose fibers with ionic liquids. Chem Fiber Int 06:342–343
Hoh GLK, Barlow DO, Chadwick AF, Lake DB, Sheeran SR (1963) Hydrogen peroxide oxidation of tertiary amines. J Am Oil Chem Soc 40:268–271. doi:10.1007/BF02633687
Kreher UP, Rosamilia AE, Raston CL, Scott JL, Strauss CR (2004) Self-associated, “distillable” ionic media. Molecules 9:387–393
Licandro E, Maiorana S, Papagni A, Pryce M, Gerosa AZ, Riva S (1995) Asymmetric synthesis of (−)-(2R, 6R)-2, 6-dimethylmorpholine. Tetrahedron Asymmetry 6:1891–1894. doi:10.1016/0957-4166(95)00245-K
Malz F, Jäger C, Yoneda Y, Kosma P, Rosenau T (2007) Synthesis of methyl 4′-O-methyl-β-d-cellobioside-13C12 from d-glucose-13C6. Part 2: solid state NMR studies. Carbohydr Res 342:65–70. doi:10.1016/j.carres.2006.11.005
Marini I, Firgo H, Kalt W (1994) Lenzing Lyocell. Lenzinger Ber 74:53–57
Ouchi M, Inoue Y, Kanzaki T, Hakushi T (1984) Molecular design of crown ethers. 1. Effects of methylene chain length: 15- to 17-crown-5 and 18- to 22-crown-6. J Org Chem 49:1408–1412. doi:10.1021/jo00182a017
Potthast A, Rosenau T, Buchner R, Röder T, Ebner G, Bruglachner H et al (2002) The cellulose solvent system N,N-dimethylacetamide/lithium chloride revisited: the effect of water on physicochemical properties and chemical stability. Cellulose 9:41–53. doi:10.1023/A:1015811712657
Potthast A, Rosenau T, Kosma P (2006) Analysis of oxidized functionalities in cellulose. Adv Polym Sci 205:1–48
Radeglia R, Andersch J, Schroth W (1989) Zum dynamischen Strukturverhalten des Dimethylamin–Kohlendioxid–Komplexes (Dimcarb). Z Naturforsch 44b:181–186
Richarz W, Lutz M, Guyer A (1959) Katalytische Aminierung von Methanol. Helv Chim Acta 42:2120–2212. doi:10.1002/hlca.19590420649
Richarz W, Lutz M, Guyer A (1962) Katalytische Aminierung von Methanol. 2. Mitteilung. Helv Chim Acta 45:2154–2161. doi:10.1002/hlca.19620450650
Rosenau T, Potthast A, Sixta H, Kosma P (2001) The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (lyocell process). Prog Polym Sci 26:1763–1837. doi:10.1016/S0079-6700(01)00023-5
Rosenau T, Potthast A, Rohrling J, Hofinger A, Sixta H, Kosma P (2002) A solvent-free and formalin-free Eschweiler–Clarke methylation for amines. Synth Commun 32:457–459. doi:10.1081/SCC-120002131
Rosenau T, Hofinger A, Potthast A, Kosma P (2003) On the conformation of the cellulose solvent N-methylmorpholine-N-oxide (NMMO) in solution. Polymer 44:6153–6158. doi:10.1016/S0032-3861(03)00663-3
Ruhoff JR, Reid EE (1937) A series of aliphatic dimethyl amides. J Am Chem Soc 59:402. doi:10.1021/ja01281a054
Striegel AM (1997) Theory and applications of DMAc/LiCl in the analysis of polysaccharides. Carb Polym 34:267–274. doi:10.1016/S0144-8617(97)00101-X
Sun N, Swatloski RP, Maxim ML, Rahman M, Harland AG, Haque A et al (2008) Magnetite-embedded cellulose fibers prepared from ionic liquid. J Mater Chem 18:283–290. doi:10.1039/b713194a
Swatloski RP, Spear SK, John D, Holbrey JD, Rogers RD (2002) Dissolution of cellulose with ionic liquids. J Am Chem Soc 124:4974–4975. doi:10.1021/ja025790m
Wu J, Zhang J, Zhang H, He J, Ren Q, Guo M (2004) Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 5:266. doi:10.1021/bm034398d
Yoneda Y, Kawada T, Rosenau T, Kosma P (2005) Synthesis of methyl 4′-O-methyl-β-cellobioside-13C12 from d-glucose-13C6. Part 1: Reaction optimization and synthesis. Carbohydr Res 340:2428–2435. doi:10.1016/j.carres.2005.08.003
Zhu S, Wu Y, Chen Q, Yu Z, Wang C, Jin S et al (2006) Dissolution of cellulose with ionic liquids and its application: a mini-review. Green Chem 8:325–327. doi:10.1039/b601395c
Acknowledgement
The financial support by the Austrian Fonds zur Förderung der wissenschaftlichen Forschung, project P-17426 is gratefully acknowledged. The authors would like to thank Dr. Andreas Hofinger, Department of Chemistry at the University of Agricultural Sciences Vienna, for recording the NMR spectra. Furthermore, the authors are indebted to Dr. Hiroshi Kamitakahara, Graduate School of Agriculture, Kyoto University, and to Dr. Toshinari Kawada, Faculty of Agriculture, Kyoto Prefectural University, for their support during this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adelwöhrer, C., Yoneda, Y., Takano, T. et al. Synthesis of the perdeuterated cellulose solvents N-methylmorpholine N-oxide (NMMO-d11 and NMMO-15N-d11), N,N-dimethylacetamide (DMAc-d9 and DMAc-15N-d9), 1-ethyl-3-methylimidazolium acetate (EMIM-OAc-d14) and 1-butyl-3-methylimidazolium acetate (BMIM-OAc-d18). Cellulose 16, 139–150 (2009). https://doi.org/10.1007/s10570-008-9241-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-008-9241-5