Abstract
Hydrogen peroxide can be catalyzed to bleach cotton fibers at temperatures as low as 30°C by incorporating dinuclear tri-μ-oxo bridged manganese(IV) complex of the ligand 1,4,7-trimethyl-1,4,7-triazacyclononane (MnTACN) as the catalyst in the bleaching solution. The catalytic system was found to be more selective under the conditions applied than the non-catalytic H2O2 system, showing better bleaching performance while causing slightly lower decrease in degree of polymerization (DP) of cellulose. In order to gain fundamental knowledge of the bleach effect on cotton fibers and cellulose as its main component, especially after catalytic bleaching, X-ray Photoelectron Spectroscopy (XPS) was used to study surface chemical effects. The Washburn method was applied to investigate wetting properties, and liquid porosity was used to obtain pore volume distribution (PVD) plots. Parallel analyzes performed on model cotton fabric, i.e. “clean” cotton fabric stained with morin - a pigment regularly found in native cotton fiber, helped to differentiate between pigment oxidation and other bleaching effects produced on the (regular) industrially scoured cotton fabric. Bleaching was not limited to the chemical action but also affected cotton fiber capillary parameters most likely due to the removal of non-cellulosic materials as well as chain-shortened cellulose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of pretreatments such as scouring and bleaching is to improve the absorbency and whiteness of cotton by removing the naturally occurring impurities, including colored matter i.e. pigments. The current most common industrial bleaching agent is hydrogen peroxide owing to its simplicity of use and eco-friendly nature. The 60% of annual world production of hydrogen peroxide is used for the bleaching of cellulose-based materials i.e. pulp/paper or textiles. If the bleaching parameters are carefully controlled, hydrogen peroxide causes low damage to cellulose, at the same time imparting permanent whiteness to the bleached material (Cardamone and Marmer 1995).
In bleaching of cotton, hydrogen peroxide is commonly applied during 2–5 h at pH 10.5–12 and temperatures close to boil, the conditions that pose a serious problem due to possible radical reactions of the bleaching compounds with the fiber. These reactions can lead to a decrease in the degree of polymerization of cellulose and eventually to a drop in tensile strength, especially in the presence of particular metal ions which act as activators for hydrogen peroxide (Meyer et al. 1987). Additionally, alkaline conditions negatively influence the effluent treatment and the temperatures needed for the process impact the cost. The bleaching parameters, e.g. time, temperature, and concentration of chemicals present in the bleaching bath, are all interrelated. Thus, hydrogen peroxide can effectively bleach cotton at low temperatures, but long times and higher chemical concentrations are required.
Chemical processes are nowadays under increasing pressure to be environmentally safe, cost-effective and energetically efficient. Decreasing the temperature of the bleaching process is an important challenge that will undoubtedly lead to general savings in energy consumption. For hydrogen peroxide cotton bleaching at low temperatures (≤40°C) and short treatment times, the processes on the basis of oxidative catalysts are a promising alternative. The use of catalysts could also result in lowering the concentration of chemicals used, compared to the conventional process. Generally, manganese containing compounds are preferred as possible catalysts in bleaching because of their environmental digestibility. Today, probably the most effective catalysts for stain oxidation by hydrogen peroxide at low temperatures are manganese complexes derived from 1,4,7-trimethyl-1,4,7-triazacyclononane and related ligand systems (Hage et al. 1994; Hage and Lienke 2006). Up to now research on the application of these catalysts has mainly been done in detergent application and paper/pulp bleaching (Hage and Lienke 2006; Patt et al. 1997), showing that the catalytic route can give improved stain removal and delignification.
The dinuclear tri-μ-oxo bridged manganese(IV) complex of the ligand 1,4,7-trimethyl-1,4,7-triazacyclononane complex (Fig. 1a, MnTACN) was the first bleach catalyst employed in commercial detergent products for the purpose of laundry cleaning (Favre et al. 1991, 1993; Hage et al. 1994). The compound was first published in 1988 as a model for manganese-containing enzymes by Wieghardt and co-workers (Wieghardt et al. 1988). According to the examples given in the original patent, the bleaching activity at 40°C on tea-model stains is very high (Favre et al. 1991). Other stains such as wine, fruit, and curry stains were also bleached efficiently, in all cases using hydrogen peroxide. The efficiency of tea stain bleaching is optimal between pH 9 and 11. However, the detergent product containing this catalyst was withdrawn from the market (Verrall 1995) after it was alleged that the product yields increased fabric- and dye-damage after several washing cycles. The catalyst is still applied in various machine-dishwashing products and is responsible for superior removal of tea residues.
Although the application of this catalyst in the detergent formulation has not been quite successful in laundry cleaning, it is important to underline that the bleaching of raw cotton is very different from laundry cleaning. Besides general requirements for the application of a bleach catalyst in both laundry cleaning and raw cotton bleaching, i.e. good bleaching activity combined with negligible fiber damage, the processing conditions (e.g. process speed, dosage and number of repeating processing steps) as well as the nature of fabric subjected to bleaching are significantly different. As a consequence, additional requirements for the application of the catalyst in laundry bleaching are: (i) no interactions with dye molecules on cotton and (ii) the inhibition of dye transfer (staining of white textiles by dye molecules coming off the colored fabrics) by bleaching the migrating dyes in the wash liquor. Therefore, the potential application of catalytic oxidation in laundry bleaching involves two types of action: a homogeneous reaction of dye transfer inhibition and a heterogeneous bleaching with a wide variety of stains (hydrophilic as well as oily), without fiber or color damage after a number of repeated washing cycles (Dannacher 2006). Moreover, the detergent producers should always anticipate a possible overdosing done often by consumers in household laundry cleaning. These all pose relatively complex requirements with regard application of bleaching catalysts in laundry cleaning entailing rather high catalyst selectivity.
On the contrary, important limitations related towards application of catalysts in detergent formulations such as, for example, dye transfer inhibition and color damage, are not an issue in catalytic bleaching of raw cotton. In addition to this, raw cotton is subjected only once to a bleaching process, different from laundry bleaching where the goods are repeatedly washed leading to increased fiber damage. The overdosing of catalytic bleach system that can lead to fiber damage in laundry cleaning can easily be avoided during bleaching of raw cotton in industrial conditions where the process is performed under strictly controlled conditions. Based on these considerations, the application of the catalysts in industrial bleaching of raw cotton seems to be very promising.
To our knowledge, no scientific literature, apart from our previous work, related to the use of the catalyst MnTACN in bleaching of raw cotton has been available yet and no industrial bleaching process has emerged. Our earlier results have shown that short-time bleaching of raw cotton with hydrogen peroxide can be accomplished at temperatures as low as 30°C by incorporating the MnTACN complex as the catalyst in the bleaching solution (Topalovic et al. 2004, 2005b). The optimum pH for catalytic bleaching is at 10.5, which is lower than in the conventional hydrogen peroxide bleaching processes, whereas the catalytic amount of MnTACN as low as 10 μM (0.016% o.w.f) is typically used.
In order to introduce an efficient, stable and reproducible catalytic bleaching process in industry, reliable information about hydrogen peroxide bleaching in the presence of MnTACN is of ultimate importance. Aiming to establish the overall mechanisms and in particular the factors governing reactivity and catalysis, we have investigated, in a homogeneous model system, the mechanism of catalytic hydrogen peroxide oxidation of pigments present in native cotton fiber. Bleaching has scarcely been investigated as regards the nature of pigments present in cotton fibers. It was reported (Sadov et al. 1978) that morin (3,5,7,2′,4′-pentahydroxylflavone, Fig. 1b) or gossypetin (3,5,7,8,3′4′-hexahydroxyl flavone) are main representatives of colored matter in cotton. In order to mimic the natural cotton colored matter, we have chosen morin as a substrate to study catalytic discoloration in a homogeneous model system. The morin oxidative degradation reaction has been monitored by UV–Vis spectrophotometry and the influence of the pH is examined (Topalovic et al. 2005a). Moreover, this study has pointed out the possibility of replacing H2O2 with O2 as terminal oxidant in the catalytic bleaching system (Topalovic et al. 2006). In this way, by excluding transport phenomena and with the assumption that the mechanism of oxidation of colored matter present in cotton fiber is similar to that in a homogeneous system, it was possible to provide direct information about the kinetics and reaction mechanisms at molecular level.
Nevertheless, the bleaching of cotton takes place in a heterogeneous system, which is naturally more complex than a homogeneous model system studied; so phenomena other than chemical reaction between active bleaching species and coloring matter present in fibers can also play an important role. Taking this into account, in the present paper we focus on the effects of catalytic bleaching on cotton fiber and cellulose as its main component, using a regular (industrially scoured) cotton fabric as well as model cotton fabric, which has been prepared by staining with morin. This approach provided the tool to differentiate between pigment discoloration and other phenomena by examining the chemical and physical effects on cotton fiber caused by catalytic bleaching. Parallel to the effectiveness of bleaching and fiber chemical damage, the wetting properties, such as porosity and contact angle, are considered as well. Surface chemical changes are examined by X-ray Photoelectron Spectroscopy (XPS) analysis. The results show that cotton cellulose fibers are modified both chemically (oxidation and hydrolysis) and physically (porosity) during catalytic bleaching. Since the purpose of bleaching is to improve cotton whiteness while preventing or at least minimizing degradation, the interrelationship between the fiber chemical damage, chemical surface changes, wetting properties and pore structure has been established, showing satisfactory bleach performance of the investigated catalytic bleaching system.
Experimental
Materials
Catalyst MnTACN (Wieghardt et al. 1988, Fig. 1a) was provided by Unilever R&D (the Netherlands). Hydrogen peroxide (30 vol.%), NaOH (extra pure), NaHCO3 (extra pure) were obtained from Merck (Germany). Morin (Wu et al. 1995, Fig. 1b), supplied by Aldrich, was used in the production of model fabric. Demineralized water was used throughout this study.
For bleaching experiments, we used both the “as received” and model cotton fabric. The “as received” cotton fabric (100% cotton woven plain fabric with a weight of unit area 105 g m−2) was previously desized and double scoured under industrial conditions (sample b, Table 1). The “as received” cotton fabric, desized and scoured once (sample a, Table 1), served as a reference only. Both fabrics were kindly supplied by Vlisco B.V. (the Netherlands).
Bleaching and staining procedure
Bleaching experiments with both regular (denominated as the “as received”) and model cotton fabric were carried out in 300 ml wide neck Erlenmeyer flasks that were placed in a shaking water bath SW 21 (Julabo, Germany) in which the temperature could be controlled to ±0.2°C. Each flask was filled with a buffer solution and once the target temperature was achieved, a cotton fabric sample was introduced and the required amounts of H2O2 (30 vol.%) and 1 mM stock solution of MnTACN were added to make a final volume of 200 ml. In the experiments where H2O2 was replaced with O2, the bleaching solution was purged with air throughout the bleaching experiments. In each bleaching experiment 3 fabric strips with a total weight of 10 g were used. The conditions were as follows: liquor ratio, 20:1; [MnTACN], 10 μM (0.016% o.w.f.); [H2O2], 0.1 M (6.8% o.w.f.); NaHCO3/NaOH buffer pH 10.5, 30°C, 150 rpm. After 15 min the samples were removed, rinsed thoroughly twice in hot water and once in cold water, and dried at room temperature over night.
The main idea of producing the model cotton fabric was to take sufficiently white cotton fabric (which has most of the impurities removed) and to stain it in a controlled way with a pigment (morin) that mimics the natural cotton colored impurities. Accordingly prepared model fabric mimics raw (unbleached) cotton fabric. The “as received” double scoured cotton fabric (sample b, Table 1) was used to produce the model fabric. To remove the colored matter thoroughly, bleaching with H2O2 was carried out for 30 min under the conditions that are characteristic for the conventional hydrogen peroxide bleaching process: liquor ratio, 20:1; [H2O2], 0.1 M (6.8% o.w.f.); NaHCO3/NaOH buffer pH 11, 70°C. The bleached cotton fabric sample (sample g, Table 1) was subsequently stained with morin (sample h, Table 1). The staining procedure was as follows: liquor ratio, 20:1; [morin], 0.6 g/L (1.2% o.w.f.); [NaCl], 2.5 g/L (5% o.w.f.); NaHCO3/NaOH buffer pH 11, 30°C; 60 min; 130 rpm. After staining, the samples were rinsed twice in cold water for 60 min at a liquor ratio of 160:1.
The sampling design used for the bleaching/staining experiments as well as for the subsequent whiteness measurements, DP measurements, XPS analysis, wetting measurements and PVD analysis is given by Scheme 1.
Whiteness measurements
Bleaching results were evaluated by measuring the whiteness index (WI) of the fabric samples with a spectrophotometer 968 (X-Rite, USA) using D65 illuminant and 10° observer. Each sample was folded twice to give an opaque sample with four plies and sixteen measurements were carried out at different fabric spots. The colorimetric coordinates of the studied sample (X, Y and Z) were used to calculate chromaticity coordinates (x, y) and to determine average value of WI applying the CIE whiteness formula (CIE 1986). (Eq. 1):
where x 0 and y 0 are the coordinates of the achromatic point for the given illuminant. Values x 0 and y 0 for D65/10° are 0.3138 and 0.3310, respectively. The formula has the following limit: 40 < W < (5Y – 280), and can not be applied outside this range. All results were checked and confirmed being within the limits of the formula.
Degree of polymerization
In order to establish the amount of fiber damage caused by catalytic bleaching, the degree of polymerization (DP) of cotton fibers based on fluidity (F) was measured before and after different bleaching treatments. The fluidity measurements have been performed using cuprammonium hydroxide under standard conditions (German Standard 1977). In order to express the level of damage on the cotton samples, it was considered useful to calculate the DP of the cotton samples by using Eq. 2 (Interox 1981; Malek and Holme 2003).
The fiber damage was also expressed as the damage factor (S) (also called Eisenhut’s Tendering factor, T.F.) by using Eq. 3, originally proposed by Eisenhut (1941) to determine the degree of damage in cotton after chemical treatment:
where, DP 0, DP before treatment; DP, DP after treatment. The damage factor relates damage to the change in DP by taking into account initial DPs of undamaged cotton. The categorization of S is as follows: 0.01–0.2, undamaged; 0.21–0.3, good; 0.31–0.5 satisfactory; 0.51–0.75, slightly damaged; ≥0.75, seriously damaged (Interox 1981).
In the case of cotton fabric used in this study, the threshold DP value that represents a manifestation of fiber damage is 1450. It was determined by introducing S = 0.50 (categorized as slight damage) in Eq. 3. This value corresponds well to the DP value of 1500, which results from the wide experience the provider of the cotton fabric has in testing DP and tensile strength of the same kind of fabric after bleaching.
XPS analysis
XPS analysis was performed to investigate the surface chemical changes of the cotton fibers using a PHI Quantera Scanning ESCA Microprobe spectrometer (Physical Electronics, USA). The samples were irradiated with monochromatic Al Kα X-rays (1486.6 eV) using a X-ray spot size with a diameter of 100 μm and a power of 25 W. Two different spots were analyzed per one sample and the average values were discussed. Survey spectra were recorded with a pass energy of 224 eV (stepsize 0.8 eV), from which the surface chemical compositions were determined. In addition, high-resolution carbon (1s) spectra were recorded with a pass energy of 26 eV (stepsize 0.1 eV), from which the carbon chemical states were determined. The standard take-off angle used for analysis was 45°, producing a maximum analysis depth in the range of 3–5 nm. All binding energies values were calculated relative to the carbon (C 1s) photoelectron at 285 eV applying a linear background substraction method followed by C 1s curve deconvolution into three Gaussian’s curves. Peak positions of these curves were fixed at 285.0 eV (C–C/C–H); 286.6 eV (C–O); and 288.1 eV (C=O/O–C–O) (Beamson and Briggs 1992; Chastain and King 1995).
Wetting measurements
The wetting behavior of cotton was characterized, according to the capillary-rise method, using a Processor Tensiometer K12 (software version K121) (Krüss, Germany). The evaluation of the measured data was based on modifications of the Washburn equation (Washburn 1921) for a single capillary (Eq. 4),
which results from the combination of the expression for the Laplace pressure and the Hagen-Poiseuille equation for steady flow conditions (Szekely et al. 1971; Grundke et al. 1991; Tröger et al. 1998), where σ, the liquid surface tension; θ, the solid/liquid contact angle; A, the cross-sectional area; r, the radius of the capillary; η, the liquid viscosity; ρ, the liquid density; m, the weight of the liquid that penetrates into the capillary, and t, the penetration time.
However, in the case of fiber assemblies, the geometry of the capillary system is unknown and, therefore, the factor 2/A 2 r is replaced by an unknown factor 1/c, which leads to the modified Washburn equation (Eq. 5):
The factor η/σρ 2 reflects the properties of the test liquid, and m 2/t is to be measured. The constant c reflects the capillary geometry of the sample. Theoretically, the capillary constant for a porous solid is given by (Eq. 6) (Aranberri-Askargorta et al. 2003):
where r, the average capillary radius within the porous solid; n, the number of capillaries in the sample. From the Washburn data alone, r and n cannot be calculated independently, whereas the c factor can be determined quite reproducibly. The modified Washburn equation (Eq. 5) can be applied by making the following assumptions: (1) the liquid flow is predominantly laminar in the “pore” spaces; (2) gravity is negligible; and (3) the pore structure of the porous solid or fiber assemblies is constant (Grundke et al. 1996).
In our experiment n-decane was used as a total wetting liquid (cos θ = 1) to determine capillary constant c (Krüss 1996). Both the capillary constant c and the contact angle θ were determined as an average of 5 independent measurements performed always in the warp direction on the 2 cm × 2 cm square swatches cut from different (randomly chosen) locations on the sample. For each measurement the initial slope m 2/t of the function m 2 = f(t) was determined using a second-order polynomial fit.
Analysis of pore volume distribution
Liquid porosimetry is a procedure for determining the pore volume distribution (PVD) within a porous solid matrix. The data obtained provide a direct picture of the absolute and relative contributions of each pore size to the total available free volume within a network. Each pore is sized according to its effective radius (R eff), and contribution of each size to the total free volume is the principal objective of the analysis.
An auto-porosimeter (TRI, USA) was used to measure the intra- and interyarn pore size distribution in the fabric. Miller and Tyomkin (1994) described the principles and operating procedure for liquid porosimetry in detail, which requires quantitative monitoring of the movement of liquid into or out of a porous structure. The pore volume distribution (PVD) typical data output for a single incremental liquid extrusion run is a cumulative curve that represents the amount of liquid remaining in the pores (as the pressure increased). The first derivative of this curve as a function of pore size becomes the PVD plot, showing the fraction of the free volume of cotton fabric sample made up of pores of each indicated size (R eff). The effective radius R eff of a pore is defined by the Laplace equation (Eq. 7):
wherein: σ, surface tension of the liquid; θ, advancing or receding contact angle between the solid and the liquid; and ΔP, the Laplace pressure difference across the liquid/air meniscus. The total wetting liquid (cos θ = 1) used in this study was 0.1% solution of Triton X-100 in double distilled water.
Measurements were performed in receding mode in the range of 1–800 μm on 5 swatches 2.5 cm (weft) × 3.5 cm (warp) cut randomly from a fabric sample. The PVD taken into consideration was obtained on the basis of averaging of PVDs obtained in this way. The latter were also used to calculate the average R eff of intra- or interyarn pores as mean values of peaks appearing in region of either intra- or interyarn pores.
Results and discussion
Bleaching effectiveness and fiber damage
Generally, special emphasis is given to the problem of chemical damage of cotton fiber i.e. decrease in degree of polymerization (DP) of cellulose caused by bleaching that is, apart from whiteness index (WI), a very important indicator of the process acceptability. Since oxidative damage of cellulose may occur after bleaching, the question is whether or to what degree it is actually necessary to completely oxidize the pigments present in fiber and to remove all non-cellulosic matter for satisfactory results. It is well documented in the literature that fiber damage does not reflect through a decrease in fiber tensile strength until a considerable cellulosic chain scission has occurred (Buschle-Diller et al. 1998).
It is commonly known that the DP of cellulose can be lowered by the reaction with hydrogen peroxide, as it can be transformed into an oxycellulose. This occurs because H2O2 is a non-specific fairly active oxidant and, as both pigments and cellulose are organic molecules, it attacks them readily. Lewin and Ettinger (1969) made a comprehensive study of the reaction between hydrogen peroxide and cellulose in the form of purified cotton fibers. They differentiated between the hydrogen peroxide that decomposed and the hydrogen peroxide that oxidized the cellulose, suggesting that in an alkaline solution it is the perhydroxyl radical that oxidizes cellulose at either the C2 or C3 carbon atoms. Oxidized cellulose containing the electronegative aldehyde and ketone groups which are in position β to the glucosidic linkage are susceptible, according to the β-alkoxyl elimination mechanism, to chain cleavage (Lewin 1997).
The results obtained for the WICIE, DP and S for the “as received” and model fabric after being subjected to the different bleaching treatments are compared in Table 1. When analyzing the values obtained for DP and WICIE, it can be seen that the catalytic bleaching of the “as received” fabric (sample b, DP = 1978) does not cause significant damage (sample f, DP = 1905) whilst producing satisfactory WICIE (∼82). Bleaching under the same conditions with H2O2 alone (sample e) does not reach the target value (above 80) for the WICIE, whereas producing slightly lower DP value. The samples treated by O2/MnTACN (sample d) and in buffer solution only (sample c) show negligible change in the WICIE with no change in DP (within a standard deviation of measurement).
Conventional bleaching (sample g) results in good whiteness, similar to the one obtained by catalytic bleaching, but with considerably higher fiber damage (DP = 1543). As this sample has been used to produce the model fabric, all DP values obtained for model fabric are considerably lower when compared to the “as received” fabric. Regarding whiteness (WICIE) of the untreated model fabric (sample h), staining with morin produces WICIE ∼52, making it similar to raw unbleached cotton.
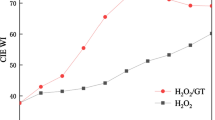
When the model fabric (sample h) is bleached in the same systems as the “as received” double scoured fabric, the DP decreases from 1361 till 1200 approximately (sample j, sample k, sample l). Therefore we can conclude that, although causing approximately the same (if not lower) fiber chemical damage, the H2O2/MnTACN bleaching is more effective in terms of WICIE produced for both fabrics tested, the “as received” and model fabric (∼82, sample f; and ∼81, sample l) than the bleaching with H2O2 alone (∼79, sample e; and ∼63, sample k). Moreover, this indicates that different species are operative in the pigment discoloration and fiber damage (i.e. oxidation of cellulose) when using the H2O2/MnTACN system instead of H2O2 alone.
The values obtained for the damage factor S show that both sample e and sample f can be categorized according to the degree of damage classification applied by Interox (1981) as “undamaged”. The sample g is categorized as “satisfactory”, while all other samples are considered as “slightly damaged”, except sample k that is categorized as “seriously damaged”. This confirms that H2O2/MnTACN bleaching at 30°C (sample f) produces the same whiteness as conventional bleaching at 70°C (sample g), with much lower fiber damage.
Surface chemical effects
Although being the purest form of plant cellulose with a composition of approximately 90% cellulose, cotton fiber contain non-cellulosic components which are located primarily in the cuticle and the primary cell wall, the outermost layers of the fiber (Tripp et al. 1951). Non-cellulosics in the cuticle play an important part in protecting against the environment and during fiber processing. Specific quantities of non-cellulosics in cotton fibers vary depending on varieties, growing conditions, and maturity, and have been reported to include waxes (∼6%), pectins (∼0.9%), proteins (∼1.3%), non-cellulosic polysaccharides (∼2.0%), ash, and other miscellaneous compounds (Tripp et al. 1951; Tripp and Rollins 1952). Pigments are usually present with less than 1%. Waxes and pectins are most responsible for the hydrophobicity or low water wettability of raw cotton fibers. After conventional (non-catalytic) scouring and bleaching cotton consists of 99% pure cellulose (Hartzell-Lawson and Hsieh 2000).
XPS analysis has been applied in order to reveal the effects of catalytic bleaching (H2O2/MnTACN) on the surface chemical composition of cotton and to compare these effects to other bleaching procedures (H2O2, O2/MnTACN). The main parameters measured are oxygen to carbon (O/C) atomic ratio and relative amounts of differently bound carbons (denominated C1, C2 and C3, as in Table 2).
The O/C atomic ratio is the main indicator of the extent of oxidation at the fiber surface. The O/C values of samples a–l determined by XPS, as well as relative amounts of differently bound carbons based on the high-resolution C 1s peaks, are given in Table 2. It is worthwhile noticing that the analysis depth was less than 5 nm (due to the take-off angle of 45°), so the results describe the properties of a limited number of molecular layers in the outermost fiber surface. The XPS analysis shows that the surface of cotton fibers for all samples analyzed (before and after bleaching) does not consist of pure cellulose. The all O/C ratio values obtained are much lower than the corresponding theoretical value for cellulose (0.83) because of the presence of considerable amounts of C1 carbon (i.e. carbon atoms without oxygen neighbors), which is not characteristic of pure cellulose that contains only components C2 and C3.
The results are in agreement with earlier studies of cellulosic materials (Soignet et al. 1976; Ahmed et al. 1987; Gellerstedt and Gatenholm 1999; Johansson et al. 2004; Fras et al. 2005; Mitchell et al. 2005) and the well known fact that laminar layers of waxes, proteins and pectin, which originally more or less totally conceal the cellulose backbone, cover natural cotton fibers. It was shown by (Buchert et al. 2001) that total removal of these “natural passivation layers” is very difficult indeed. A minimum surface concentration of non-cellulosic compounds is 10–20% even in “pure” cellulose materials (Ahmed et al. 1987; Belgacem et al. 1995; Buchert et al. 2001; Mitchell et al. 2005) attributed to non-removable impurities. Since the waxes consist mainly of C1 type carbon, calculated relative amount of C1 component can be taken as an indicator of their presence at the fiber surface.
Figure 2 presents the correlation between the calculated O/C atomic ratios and the relative amount of C1 carbon, calculated from XPS data, for differently treated the “as received” and model fabric (samples b–l). It is obvious that the O/C of conventionally bleached fabric (sample g) is not affected after staining with morin (sample h). Despite the different stoichiometry of cellulose (O/C = 0.83) and morin (O/C = 0.47), this result is expected owing to much lower proportion of morin compared to that of cellulose at the cotton fiber surface of the model fabric. Thus, the surface changes induced by the different treatments (samples i–l) of model fabric, clearly observable in the high-resolution C 1s spectra, in particular by H2O2/MnTACN bleaching, cannot be assigned to the oxidation of pigment morin. Likewise, XPS analysis cannot answer whether morin is bleached or removed by the treatment.
Correlation between the calculated O/C ratios and relative amounts of C–C bound carbon in samples b–l, calculated from XPS data. The arrow lines show the pathways for producing different samples. Labeling scheme used in Table 1
For samples b→c, d, e, f, g and h→i, j, k, a decrease in the C1 takes place concomitantly with a decrease in the carbon content, i.e. an increase in the O/C atomic ratio (Fig. 2). This indicates that higher concentration of oxygen originates from cellulose becoming more exposed due to the removal of non-cellulosic impurities from cotton fiber surface. For sample h→l, however, the O/C atomic ratio increases noticeably whilst the relative amount of C1 component remains approximately at the same level as obtained with samples i, j and k. This can point out to the increased oxidation of the cotton fiber surface after bleaching of the model fabric (sample h) with H2O2/MnTACN (sample l). Furthermore, the sample l contains reduced relative amount of C3 component (Table 2), suggesting that two concomitant processes could be postulated to interpret the effect of the H2O2/MnTACN activity on the cellulose fiber surface: oxidative degradation and removal of low-molecular-weight carbonyl-rich products into the bleaching solution. These observations indicate that the presence of a significant amount of (removable) non-cellulosic compounds (C1 component) at the cotton fiber surface can play a protective role to cotton cellulose, whereas in their absence more cellulose bonds are vulnerable on the “bare” surface.
Wetting properties
Contact angles on single natural fibers can be measured either directly (Le et al. 1996; Silva et al. 1999) or indirectly, using the modified Wilhelmy technique (Aranberri-Askargorta et al. 2003). However, in the case of natural fibers the Wilhelmy method is hardly applicable, owing to the wide variation in fiber perimeter and fiber shape (van Hazendonk et al. 1993). Dimensional irregularities along the cotton-fiber axis and in cross-sectional shapes complicate the measurements and thus affect the single-fiber contact angles. Greater variations in the measured contact angle might also be the result of the inhomogeneous character of the fiber surfaces and therefore varying fiber surface wettability. Hsieh (1992; 1994) compared the measured contact angles on a variety of fabrics (natural and man-made textile fibers) with the wetting properties of single fibers. The author found that contact angles derived from fabrics and single-fiber measurements are identical and that neither the existence nor the magnitude of liquid retention interferes with the contact-angle determination from fabrics. However, the error range is broader for contact angles measured on single fibers.
In order to avoid all the limitations of single-fiber measurements, we have used the capillary-rise method to measure the wettability of cotton fabric samples. Water contact angles of the “as received” and model cotton fabric as a function of bleaching in the presence and absence of the catalyst MnTACN are determined. The contact angles of similar value (50–54°) are obtained for all bleached samples as well as for the blanks (Table 3). The significant change in hydrophilicity occurs exclusively after the second scouring process (sample b) as a result of removal of a considerable amount of non-cellulosic compounds (relative amount of C1 component, Table 2). This means that after a properly done scouring process, bleaching is not critical to the contact angle. The values obtained for the contact angle are in accordance with literature data (Hartzell-Lawson and Hsieh 2000) where stated that scouring is effective in removal of non-cellulosics and improves the water wettability of cotton fibers to cos θ values between 0.49 and 1.05 (contact angle between 55.4° and 11.9°).
For the calculation of contact angles (Eq. 5), it was necessary to determine the capillary constant c for each sample. The values of this parameter are presented in Table 3. In fact, the capillary constant increases from sample a to sample l, whereas relatively high values for the samples bleached with H2O2/MnTACN (sample f and sample l) for both the “as received” and model fabric are obtained. The increase in capillary constant is likely to be the consequence of the removal of non-cellulosic impurities as indicated by the significant decrease in relative amount of C1 component in C 1s XPS spectrum (Table 2) with the exception of the bleached model fabric, i.e. samples i–l. The amplification in capillary constant observed between samples j, k and l, cannot be assigned to the removal of non-cellulosic compounds as their concentration remains nearly unchanged (see C1 in Table 2).
It can be speculated that the capillary constant for samples k and l changes noticeably because of possible losses of chain-shortened ‘hydrocellulose’ during bleaching (Zeronian and Inglesby 1995; Cardamone and Marmer 1995), which we anticipate to be associated with the fiber damage. In an attempt to check this hypothesis, the values of capillary constant are plotted against DP for the samples investigated (Fig. 3). The lines drawn between experimentally obtained results show that all samples can be set into three groups with respect appreciable changes in either DP or capillary constant. Despite a significant change in the capillary constant for the blank (sample c) with respect to the untreated sample b, the difference in DP between these two samples is negligible (line I). The samples with considerably different DP (from ∼2000 to ∼1500) and a similar capillary constant (∼8.5 × 10–7 cm5) are distributed along line II. These samples result from different treatments of the “as received” fabric (samples c, d, e and f) as well as from the treatments for obtaining model fabric (samples g and h).
Correlation between DP and capillary constant. The lines are drawn for visual aid only. Labeling scheme used in Table 1
As explained earlier, the threshold DP value that represents a manifestation of fiber damage is 1450, which is observed for the model fabric (sample h) and bleached model fabric (samples j, k and l). Nevertheless, it is important to realize that sample h is produced from sample g that has a DP value of ca. 1500. Therefore, the damage of sample h given in Table 1 reflects both: the damage occurred during the production of sample g from sample b (more severe) and the damage occurred during the production of sample h from sample g. The difference in DP between samples g and h is due to relatively long alkaline treatment (pH 11, 60 min), and not due to the action of morin. Subsequent bleaching of sample h leads to a further, but to approximately the same extent, decrease in DP regardless the bleaching procedure applied (samples j, k and l). For these samples the different values for capillary constant are however observed (line III in Fig. 3), with the maximum value of 10.2 × 10−7 cm5 obtained after bleaching with H2O2/MnTACN (sample l). A stepwise change in the capillary constant after bleaching of model fabric, as distinguished from the “as received” fabric, is perhaps not entirely surprising considering the “weakened” cellulosic material of the model fabric cotton. Based on that, we may postulate that during the bleaching of model fabric, the lowering of DP is coupled with a removal of chain-shortened ‘hydrocellulose’ which results in appreciable increase in the capillary constant.
Recent findings (Volkov et al. 2003) state that bleaching is not limited to the chemical action but also leads to a change of capillary parameters. Different wet-treatments, i.e. desizing, scouring, mercerizing or bleaching of cotton fabric, not only lead to an increase in the hydrophilicity of the cotton fiber surface due to the partial removal of the contaminants, but also increase the dimensions of the interfiber capillaries. As regard to bleaching, the additional increase in capillary size is caused by a modification of fiber occurring simultaneously with discoloration of cotton fiber pigments. Our results showing the increase in capillary constant c (Table 3), i.e. indirectly showing the increase in the average capillary radius (Eq. 6), with a removal of non-cellulosic impurities represented by C1 in C 1s XPS (Table 2), are in agreement with the results of Volkov et al. (2003).
These results however contradict with quite firmly established opinion in both textile practice and scientific literature (Hsieh et al. 1996) that most non-cellulosic materials present in cotton can be made soluble and removed with alkaline scouring, whilst the bleaching is limited to the oxidation of cotton fiber pigments. In their intention to quantify the effects of alkaline scouring, swelling, and bleaching of cotton assemblies, Hsieh et al. (1996) have found that scouring of cotton fabric, although improving water wettability and water retention, reduces the pore volume in the fabric, whereas bleaching, on the other hand, does not affect the fabric pore structure, while further improves the hydrophilicity. The authors explain the effect of bleaching by the cause of chemical changes only (i.e. oxidation) without affecting the pore structure of the fabrics.
Pore volume distribution
In order to examine possible effects of catalytic bleaching on the pore size of cotton fabric we have used the liquid porosimetry method. Woven fabrics, such as cotton fabrics used in this study, typically give bimodal distributions (Miller and Tyomkin 1994), where the larger pore sizes are supplied by the spaces between yarns (interyarn pores) and the smaller (intrayarn or interfiber) pores reflect intrayarn structure. Without going into details of theory that describes pores between fibers in fiber assemblies (see for instance Neckář and Ibrahim 2003), it can be understood that the pores between yarns or fibers express the relative amount of air or fluid found in the gaps between yarns and/or fibers. Intrafiber pores are the smallest and their distribution cannot be analyzed due to the method limitations. Neither of these pores are of exact size and there is always a distribution around an average value.
The pore volume distribution (PVD) plots for the “as received” and model fabric before and after bleaching are presented in Fig. 4. In general, these plots show the fraction of the free volume of cotton fabric samples made up of pores of each indicated size (effective radius, R eff). As it can be seen, the intrayarn pores range between 1 μm (lower limit of the instrument) and 22 μm, while interyarn pores range between 22 μm and 55 μm for both the “as received” and model fabric. The values of the average effective radius of intra- and interyarn pores for the two fabrics analyzed are presented in Fig. 5a–b.
PVD results for the “as received” (a) and model fabric (b) as consequence of bleaching with and without MnTACN catalyst. Labeling scheme used in Table 1
Average effective radius R eff of intrayarn (a) and interyarn (b) pores in the “as received” and model fabric as a function of the bleaching in the presence and absence of the MnTACN catalyst. Labeling scheme used in Table 1
Both smaller and larger pores remain distributed in the same range after bleaching in the presence and the absence of catalyst MnTACN (Fig. 4). Nevertheless, the average R eff represented by the peaks of the intra- and interyarn PVD’s, shift to the right (towards higher values) after bleaching of both the “as received” and model fabric with the H2O2/MnTACN catalytic system (Fig. 4). Figure 5 gives a quantitative observation of a change in the average R eff after bleaching in the presence and absence of MnTACN. Apparently, the effective radius of the largest fraction of both intra- and interyarn pores is enlarged after bleaching with the H2O2/MnTACN system (samples f and l), whereas it stays approximately unchanged after the bleaching with H2O2 alone (samples e and k). It is expected that the increase in pore size will facilitate fast liquid spreading in fabric which is essential for efficient subsequent wet-treatments such as dyeing and finishing.
Conclusions
The bleaching of cotton fabric with H2O2 catalyzed by the dinuclear manganese(IV) catalyst MnTACN at temperature as low as 30°C shows satisfactory bleaching performance while causing limited fiber damage. It produces the same level of whiteness as the conventional (non-catalytic) bleaching at 70°C, with considerably lower fiber damage. This confirms better selectivity of the catalyst based system in comparison with the less specific system with peroxide alone, indicating that different species are operative in the pigment discoloration and the cellulose oxidation when using the H2O2/MnTACN system.
The model fabric, obtained by staining with cotton pigment morin, provides the tool to differentiate between pigment discoloration and other phenomena by examining the chemical and physical effects on cotton fiber caused by catalytic bleaching. The surface changes are clearly observable by XPS analysis, particularly those induced by the H2O2/MnTACN bleaching. The decrease of the relative amount of aliphatic bound carbon is assigned to the removal of non-cellulosic compounds. Cellulose then becomes more exposed on the cotton fiber surface, which is indicated by an increased O/C ratio. On the “bare” surface, more cellulose bonds are vulnerable to the oxidation and consequently the chain-scission of cellulose, i.e. lowering of the DP of cellulose, is more likely to occur.
The measuring of wetting properties indicated that bleaching with the H2O2/MnTACN system is not limited to the chemical action (discoloration of pigments and oxidation of cellulose), but also affects capillarity of cotton. The observed increase in capillary constant for regular fabric is explained by the removal of non-cellulosics. Contrary to this, in case of model fabric, the relative amount of non-cellulosic impurities remains constant after applying different bleaching procedures. Due to the considerable differences in DP of cellulose in model and regular fabric, the increase of the capillary constant of model sample after bleaching is likely to be due to removal of chain-shortened ‘hydrocellulose’. The liquid porosimetry method indicates that also the fabric pore structure, i.e. the size of the pores in both the regular and model fabric, is affected after bleaching with the H2O2/MnTACN system. This is shown through the increase of the average effective radius of the largest fraction of intra- and interyarn pores.
The H2O2/MnTACN is an efficient bleaching system for cotton, not only in terms of satisfactory degree of whiteness and fiber damage obtained, but it additionally facilitates fast liquid spreading throughout the fabric, the latter being the essential property needed for subsequent wet-treatments.
Abbreviations
- MnTACN:
-
Dinuclear tri-μ-oxo bridged manganese(IV) complex of the ligand 1,4,7-trimethyl-1,4,7-triazacyclononane
- WI:
-
Whiteness index
- DP:
-
Degree of polymerization
- PVD:
-
Pore volume distribution
- XPS:
-
X-ray photoelectronic spectroscopy
References
Ahmed A, Adnot A, Grandmaison JL, Kaliaguine S, Douget J (1987) ESCA analysis of cellulosic materials. Cellul Chem Technol 21:481–492
Aranberri-Askargorta I, Lampke T, Bismarck A (2003) Wetting behavior of flax fibers as reinforcement for polypropylene. J Colloid Interface Sci 263:580–589
Beamson G, Briggs D (1992) High resolution XPS of organic polymers. The scienta ESCA database. Wiley, Chichester, UK
Belgacem MN, Czeremuszkin G, Sapieha S (1995) Surface characterization of cellulose fibres by XPS and inverse gas chromatography. Cellulose 2:145–157
Buchert J, Pere J, Johansson L-S, Campbell JM (2001) Analysis of the surface chemistry of linen and cotton fabrics. Text Res J 71:626–629
Buschle-Diller G, El Mogahzy Y, Inglesby MK, Zeronian SH (1998) Effects of scouring with enzymes, organic solvents, and caustic soda on the properties of hydrogen peroxide bleached cotton yarn. Text Res J 68:920–929
Cardamone JM, Marmer WN (1995) The whitening of textiles. In: Carr CM (ed) Chemistry of the textile industry. Blackie Academic & Professional, Chapman and Hall, New York, pp 46–101
Chastain J, King RC Jr (eds) (1995) PHI handbook of X-ray photoelectron spectroscopy, physical electronics, Eden Prairie, MN
CIE (International Commission on Illumination) (1986) Colorimetry, 2nd edn, CIE Publication 15.2. CIE Central Bureau, Vienna, p 36
Dannacher JJ (2006) Catalytic bleach: most valuable applications for smart oxidation chemistry. J Mol Catal A – Chem 251:59–176
Eisenhut O (1941) Zur Frage der Bestimmung des Schadigungswertes von Fasern, Garnen oder Geweben aus Zellulose und von Zellstoffen. Melliand Textilber 22:424–429
Favre F, Hage, R, van der Helm-Rademaker K, Koek JH, Martens RJ, Swarthoff T, van Vliet MRP (1991) Bleach activation. EP 0458397, Unilever NV
Favre F, Hage R, van der Helm-Rademaker K, Koek JH, Martens RJ, Swarthoff T, van Vliet MRP (1993) Bleach activation multinuclear manganese-based coordination complexes. US Patent 5244594, Lever Brothers Company, Division of Conopco, Inc.
Fras L, Johansson L-S, Stenius P, Laine J, Stana-Kleinschek K, Ribitsch V (2005) Analysis of the oxidation of cellulose fibres by titration and XPS. Colloids Surf, A 260:101–108
Gellerstedt F, Gatenholm P (1999) Surface properties of lignocellulosic fibers bearing carboxylic groups. Cellulose 6:103–121
German Standard (1977) DIN 54270, Teil 2
Grundke K, Boerner M, Jacobasch H-J (1991) Characterization of fillers and fibers by wetting and electrokinetic measurements. Colloids Surf 58:47–59
Grundke K, Bogumil T, Gietzelt T, Jacobasch H-J, Kwok DY, Neumann AW (1996) Wetting measurements on smooth, rough and porous solid surfaces. Prog Colloid Polym Sci 101:58–68
Hage R, Iburg JE, Kerschner J, Koek JH, Lempers ELM, Matrens RJ, Racherla US, Russell SW, Swarthoff T, van Vliet MRP, Warnaar JB, van der Wolf L, Krijnen B (1994) Efficient manganese catalysts for low-temperature bleaching. Nature 369:637–639
Hage R, Lienke A (2006) Applications of transition-metal catalysts to textile and wood-pulp bleaching. Angew Chem Int Ed 45:206–222
van Hazendonk JM, van der Putten JC, Keurentjes JTF, Prins A (1993) A simple experimental method for the measurement of the surface tension of cellulosic fibres and its relation with chemical composition. Colloids Surf, A 91:251–261
Hartzell-Lawson MM, Hsieh Y-L (2000) Characterizing the noncellulosics in developing cotton fibers. Text Res J 70:810–819
Hsieh Y-L (1992) Liquid wetting, transport, and retention properties of fibrous assemblies. I. Water wetting properties of woven fabrics and their constituent single fibers. Text Res J 62:677–685
Hsieh Y-L (1994) Wetting contact angle derivations of cotton assemblies with varying perimeters. Text Res J 64:552–553
Hsieh Y-L, Thompson J, Miller A (1996) Water wetting and retention of cotton assemblies as affected by alkaline and bleaching treatments. Text Res J 66:456–464
Interox (1981) A bleachers handbook. Solvay Interox, Houston, TX, p 57
Johansson L-S, Campbell J, Kolijonen K, Kleen M, Buchert J (2004) On surface distributions in natural cellulose fibres. Surf Interface Anal 36:706–710
Krüss (1996) Krüss users manual, K121 contact-angle and adsorption measuring system, Version 2.1, Part C. KRÜSS GmbH, Hamburg, Germany, p 11
Le CV, Ly NG, Stevens MG (1996) Measuring the contact angles of liquid droplets on wool fibers and determining surface energy components, Text Res J 66:389–397
Lewin M, Ettinger A (1969) Oxidation of cellulose by hydrogen peroxide. Cellul Chem Technol 3:9–20
Lewin M (1997) Oxidation and aging of cellulose. Macromol Symp 118:715–724
Malek RMA, Holme I (2003) The effect of plasma treatment on some properties of cotton. Iran Polym J 12:271–280
Meyer U, Kokot S, Weber R, Zuercher-Vogt I 1987, Wie entstehen Katalytschaeden? Textilveredlung 22:185–190
Miller B, Tyomkin I (1994) Liquid porosimetry: new methodology and applications. J Colloid Interface Sci 162:163–170
Mitchell R, Carr CM, Parfitt M, Vickerman JC, Jones C (2005) Surface chemical analysis of raw cotton fibres and associated materials. Cellulose 12:629–639
Neckář B, Ibrahim S (2003) Theoretical approach for determining pore characteristics between fibers. Text Res J 73:611–619
Patt R, Mielisch HJ, Odermatt J, Wieghardt K, Weyhermüller T (1997) Method for the delignification of fibrous material and use of the catalyst. US-A-0025695, 1997. [Chem Abstr 2001, 128, 49695]
Sadov F, Korchagin M, Matetsky A (1978) Chemical technology of fibrous materials. Mir Publishers, Moscow, pp 52–53
Silva JLG, Al-Qureshi HA (1999) Mechanics of wetting systems of natural fibres with polymeric resin. J Mater Process Tech 92–93:124–128
Soignet DM, Berni RJ, Benerito RR (1976) ESCA – a tool for studying treated textiles. J Appl Polym Sci 20:2483–2495
Szekely J, Neumann AW, Chuang YK (1971) The rate of capillary penetration and the applicability of the Washburn equation. J Colloid Interface Sci 35:273–278
Topalovic T, Nierstrasz VA, Warmoeskerken MMCG (2004) The assesment of kinetics of cotton catalytic bleaching. 4th AUTEX Conference, World Textile Conference Proceedings, 22–24 June 2004, ENSAIT Roubaix, France. CD-ROM, ISBN 2-9522440-0-6, Paper O-COL11, p 1–7
Topalovic T, Nierstrasz VA, Hage R, Browne WR, Feringa BL, Warmoeskerken MMCG (2005a) Model system for mechanistic study of catalytic bleaching of cotton. 5th AUTEX Conference Proceedings, 27–29 June, 2005, Portorož, Slovenia, pp 916–923
Topalovic T, Nierstrasz VA, Warmoeskerken MMCG (2005b) Efficient bleaching of cotton with hydrogen peroxide using a new [Mn2O3(tmtacn)2]2+ catalyst reaction system. 1st South East European Congress of Chemical Engineering (SEECChE1), Book of abstracts, September 25–28, 2005, Belgrade, Serbia and Montenegro, ISBN 86-905111-0-5, p 22
Topalovic T, Nierstrasz VA, Hage R, Browne WR, Feringa BL, Warmoeskerken MMCG (2006) Oxygen activation by [Mn2O3(tmtacn)2]2+ in the catalysed flavonoids oxidation under ambient conditions. Netherlands’ Catalysis and Chemistry Conference (NCCC 2006), Book of abstracts, March 6–8, 2006, Noordwijkerhout, The Netherlands, Paper O-75, p 139
Tripp VW, Moore AT, Rollins ML (1951) Some observations on the constitution of the primary wall of the cotton fiber, Text Res J 21:886–894
Tripp VW, Rollins ML (1952) Morphology and chemical composition of certain components of cotton fiber cell wall, Anal Chem 24:1721–1728
Tröger J, Lunkwitz K, Grundke K, Bürger W (1998) Determination of the surface tension of microporous membranes using wetting kinetics measurements. Colloids Surf, A 134:299–304
Verrall M (1995) Unilever consigns manganese catalyst to the back-burner. Nature 373:181
Volkov VA, Bulushev BV, Ageev AA (2003) Determination of the capillary size and contact angle of fibers from the kinetics of liquid rise along the vertical samples of fabrics and nonwoven materials. Colloid J 65:523–525
Washburn EW (1921) The dynamics of capillary flow. Phys Rev, 2nd Series 27:273–283
Wieghardt K, Bossek U, Nuber B, Weiss J, Bonvoisin J, Corbella M, Vitols SE, Girerd JJ (1988) Synthesis, crystal structures, reactivity, and magnetochemistry of a series of binuclear complexes of manganese(II), -(III), and -(IV) of biological relevance. The crystal structure of [L’MIV(μ-O)3MIVL’](PF6)2·H2O containing an unprecedented short Mn···Mn distance of 2.296 Å. J Am Chem Soc 110:7398–7411
Wu T-W, Fung K-P, Zeng L-H, Wu J, Hempel A, Grey AA, Camerman N (1995) Molecular properties and myocardial salvage effects of morin hydrate. Biochem Pharmacol 49:537–543
Zeronian SH, Inglesby MK (1995) Bleaching of cellulose by hydrogen peroxide. Cellulose 2:265–272
Acknowledgements
The authors gratefully acknowledge Mr. A.H.J. Tijhuis for arranging the DP analysis at Vlisco Helmond B.V. The work is financially supported by the E.E.T. program (Project Nr. EET01108). E.E.T. (Economy, Ecology and Technology) is a program of the Dutch Ministry of Economic Affairs, the Dutch Ministry of Education, Culture and Science (OCenW) and the Dutch Ministry of Housing, Spatial Planning and the Environment (VROM). The Textile Technology Group at the University of Twente acknowledges the financial support of the Foundation Technology of Structured Materials in the Netherlands and the Dutch Ministry of Economic Affairs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Topalovic, T., Nierstrasz, V.A., Bautista, L. et al. Analysis of the effects of catalytic bleaching on cotton. Cellulose 14, 385–400 (2007). https://doi.org/10.1007/s10570-007-9120-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-007-9120-5