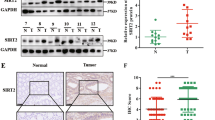
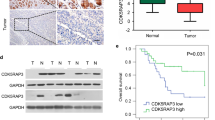
Abstract
Unlike angiogenesis in normal tissues, tumor angiogenesis is typically dysregulated, during which the HIF1/VEGFA signaling pathway plays a pivotal role. Solid tumors generate immature vessels, which promote tumor progression and treatment resistance. NSD1 can di-methylate histone 3 lysine 36 and regulate transcription factors binding to the promoters of various genes. However, the role of NSD1 in tumorigenesis remains elusive. Here, we evaluated the relationship between NSD1 signaling and HIF1 signaling. It was found that NSD1 transcriptionally regulates HIF1α expression by recruiting STAT3 molecule into the HIF1α promoter. In vivo xenograft experiments further confirmed that HIF1α and STAT3 maintenance is essential for NSD1-mediated tumor progression and angiogenesis. Therefore, the NSD1/STAT3/HIF1α signaling pathway may be a novel and effective treatment target for ESCA.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Bianco-Miotto T, Chiam K, Buchanan G, Jindal S, Day TK, Thomas M, et al. Global levels of specific histone modifications and an epigenetic gene signature predict prostate cancer progression and development. Cancer Epidemiol Biomark Prev. 2010;19(10):2611–22.
Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82.
Choufani S, Cytrynbaum C, Chung BH, Turinsky AL, Grafodatskaya D, Chen YA, et al. NSD1 mutations generate a genome-wide DNA methylation signature. Nat Commun. 2015;6:10207.
Chu G, Li Y, Dong X, Liu J, Zhao Y. Role of NSD1 in H2O2-induced GSTM3 suppression. Cell Signal. 2014;26(12):2757–64.
de Heer EC, Jalving M, Harris AL. HIFs, angiogenesis, and metabolism: elusive enemies in breast cancer. J Clin Invest. 2020;130(10):5074–87.
De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–74.
Denko NC. Hypoxia, HIF1 and glucose__metabolism in the solid tumour. Nat Rev Cancer. 2008;8(9):705–13.
Deshpande AJ, Deshpande A, Sinha AU, Chen L, Chang J, Cihan A, et al. AF10 regulates progressive H3K79 methylation and HOX gene expression in diverse AML subtypes. Cancer Cell. 2014;26(6):896–908.
Enzinger PC, Mayer RJ. Esophageal Cancer. N Engl J Med. 2003;349:2241–52.
Eswarappa SM, Fox PL. Antiangiogenic VEGF-Ax: a new participant in tumor angiogenesis. Cancer Res. 2015;75(14):2765–9.
Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2(10):803–11.
He F, Xiao H, Cai Y, Zhang N. ATF5 and HIF1alpha cooperatively activate HIF1 signaling pathway in esophageal cancer. Cell Commun Signal. 2021;19(1):53.
Hollink IH, van den Heuvel-Eibrink MM, Arentsen-Peters ST, Pratcorona M, Abbas S, Kuipers JE, et al. NUP98/NSD1 characterizes a novel poor prognostic group in acute myeloid leukemia with a distinct HOX gene expression pattern. Blood. 2011;118(13):3645–56.
Huang H, Howard CA, Zari S, Cho HJ, Shukla S, Li H, et al. Covalent inhibition of NSD1 histone methyltransferase. Nat Chem Biol. 2020;16(12):1403–10.
Jimenez-Valerio G, Casanovas O. Angiogenesis and metabolism: entwined for therapy resistance. Trends Cancer. 2017;3(1):10–8.
Kasper S, Schuler M. Targeted therapies in gastroesophageal cancer. Eur J Cancer. 2014;50(7):1247–58.
Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129(3):465–72.
Morera L, Lubbert M, Jung M. Targeting histone methyltransferases and demethylases in clinical trials for cancer therapy. Clin Epigenetics. 2016;8:57.
Ostronoff F, Othus M, Gerbing RB, Loken MR, Raimondi SC, Hirsch BA, et al. NUP98/NSD1 and FLT3/ITD coexpression is more prevalent in younger AML patients and leads to induction failure: a COG and SWOG report. Blood. 2014;124(15):2400–7.
Pawlus MR, Wang L, Hu CJ. STAT3 and HIF1alpha cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene. 2014;33(13):1670–9.
Qiao Q, Li Y, Chen Z, Wang M, Reinberg D, Xu RM. The structure of NSD1 reveals an autoregulatory mechanism underlying histone H3K36 methylation. J Biol Chem. 2011;286(10):8361–8.
Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371(26):2499–509.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–32.
Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda). 2004;19:176–82.
Semenza GL. Hypoxia-Inducible Factor 1 (HIF-1) Pathway. Sci STKE. 2007;407:cm8.
Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29(5):625–34.
Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5(6):437–48.
Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048.
Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60(2):203–12.
Wagner EJ, Carpenter PB. Understanding the language of Lys36 methylation at histone H3. Nat Rev Mol Cell Biol. 2012;13(2):115–26.
Wang GG, Cai L, Pasillas MP, Kamps MP. NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat Cell Biol. 2007;9(7):804–12.
Wang H, Byfield G, Jiang Y, Smith GW, McCloskey M, Hartnett ME. VEGF-mediated STAT3 activation inhibits retinal vascularization by down-regulating local erythropoietin expression. Am J Pathol. 2012;180(3):1243–53.
Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17(11):1359–70.
You L, Wu W, Wang X, Fang L, Adam V, Nepovimova E, et al. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med Res Rev. 2020.
Zanotelli MR, Reinhart-King CA. Mechanical forces in tumor angiogenesis. Adv Exp Med Biol. 2018;1092:91–112.
Zhang S, Zhang F, Chen Q, Wan C, Xiong J, Xu J. CRISPR/Cas9-mediated knockout of NSD1 suppresses the hepatocellular carcinoma development via the NSD1/H3/Wnt10b signaling pathway. J Exp Clin Cancer Res. 2019;38(1):467.
Funding
This study has been supported by the double first class construction project of Tongji Medical College of Huazhong University of science and technology—research clinician funding scheme, 3011540027 and 5001540077, and Natural Science Foundation of Hubei Province, 2016CFB550.
Author information
Authors and Affiliations
Contributions
HF mainly performed the experiment. HF, XH, and CY helped with cell culture, solution preparation, reagents ordering, and in vivo experiments. All authors contributed to collection and grading of clinical samples. ZN provided important comments and opinions for the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Huazhong University of Science and Technology Ethics Committee. All clinic samples were obtained from ESCA patients, and the written informed consent of all patients was received.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Supplementary Figure 1
NSD1 acted as an oncogene in esophageal cancer. a–d Representative images (scale = 200 um) from transwell assays (a, c) and wound healing assays (b, d). e Illustration of colony formation assays. f Representative image of the IHC assay for subcutaneous xenograft tumors, scale = 200 μm. g Statistical analyses for microvessel density of subcutaneous xenograft tumors; **p < 0.05 (PNG 6040 kb)

Supplementary Figure 2
NSD1 regulated the HIF1/VEGFA signaling pathway. a Relationship between VEGFA and NSD1 expression in esophageal cancer, based on the TCGA database. b, c Immunoblots showing protein expression of cells ectopically expressing vector or NSD1 (b) or NSD1 R1984Q (c). d Immunoblots showing protein expression of cells ectopically expressing shnc or shNSD1. e, f Expression levels of VEGFA in conditional media (CM) of cells stably expressing vector or NSD1 in normoxic (e) and hypoxic (f) environments, as revealed by ELISA; **p < 0.05. g Proliferation activities of HUVECs cultured in CM of cells ectopically expressing vector or NSD1 in normoxia, as revealed by CCK8 assays; **p < 0.05. h Proliferation activities of HUVECs cultured in CM of cells ectopically expressing shnc or shNSD1 under normoxia, as revealed by CCK8 assays; **p < 0.05 (PNG 904 kb)

Supplementary Figure 3
NSD1 regulated tumor angiogenesis in vitro. a, b Representative images of tube formation assays for HUVECs cultured in CM from cells ectopically expressing shnc or shNSD1 under normoxic (a) and hypoxic (b) conditions, scale = 200 μm. c, d Representative images of trans-well assays for HUVECs cultured in CM of cells ectopically expressing shnc or shNSD1 under normoxia (c) and hypoxia (d) conditions, scale = 200 μm (PNG 3376 kb)

Supplementary Figure 4
STAT3 acted as a co-activator of NSD1-mediated tumor process. a GSEA analysis of the GSE100942 dataset. b Immunoblots showing the expression levels of indicated proteins in cells expressing shnc or shNSD1. c Immunoblots demonstrating successful construction of cells expressing shnc or shSTAT3. d Representative IHC assay image of subcutaneous xenograft tumors, scale = 200 μm. e Immunoblots showing successful construction of indicated cells. f representative tumor images; scale = 1 cm. g Immunoblots of subcutaneous xenograft tumors showing expression levels of indicated proteins (PNG 2711 kb)
Supplementary Table 1
(DOCX 20 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, F., Xiao, H., Cai, Y. et al. NSD1 promotes esophageal cancer tumorigenesis via HIF1α signaling. Cell Biol Toxicol 39, 1835–1850 (2023). https://doi.org/10.1007/s10565-022-09786-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-022-09786-2