Abstract
Extracellular vesicles (EVs) play novel roles in homeostasis through cell-to-cell communication in human airways via transferring miRNAs. However, the contribution of EV miRNAs to pulmonary phenotypic homeostasis is not clearly understood. Hence, the aim of this study was to elucidate the functional role of miRNAs obtained from epithelium-derived EVs in lung fibrogenesis. Pulmonary fibrosis was induced by exposure of polyhexamethylene guanidine phosphate (PHMG-p)-instilled mice. In histopathological changes, a clear phenotypic change was observed in bronchial epithelium. For figuring out the role of EVs derived from conditioned media of untreated cells (EV-Con) and PHMG-p-treated BEAS-2B (EV-PHMG), significant increase in EVs released from PHMG-p-treated BEAS-2B was detected. Functional analysis with targets of differentially expressed miRNAs in EVs was annotated to epithelial–mesenchymal transition (EMT). Especially, the most abundant miRNA, miR-451a, was downregulated in EV of PHMG-p-treated BEAS-2B cells. We found that odd-skipped related 1 (OSR1) was a putative target for miR-451a, which had been known as a transcription factor of several fibrosis-associated genes. Transfer of decreased miR-451a via EV-PHMG upregulated OSR1 and induced EMT compared to Con-EV-treated cells. In pulmonary fibrosis mice, miR-451a levels were significantly reduced in EV derived from bronchoalveolar lavage fluid and OSR1 expression was increased in lung tissues of mice with PHMG-p exposure. MiR-451a-transfected EVs markedly alleviated fibrogenesis in the PHMG-p-exposed lungs. Low level of miR-451a in EVs modulated EMT and fibrogenesis in recipient cells by increasing OSR1 levels in vitro and in vivo. Our results suggest that transferring EV miR-451a induces anti-fibrotic autocrine effect by downregulating its target, OSR1 maintaining pulmonary homeostasis disrupted by PHMG-p exposure, which can be a potential therapeutic target.
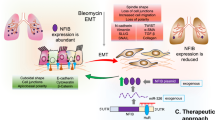
Graphical abstract








Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Alipoor SD, Mortaz E, Garssen J, Movassaghi M, Mirsaeidi M, Adcock IM. Exosomes and exosomal miRNA in respiratory diseases. Mediators Inflamm. 2016;2016:5628404.
Bagnato G, Roberts WN, Roman J, Gangemi SA. Systematic review of overlapping microrna patterns in systemic sclerosis and idiopathic pulmonary fibrosis. Eur Respir Rev. 2017;26(144):160125.
Chanda D, Otoupalova E, Smith SR, Volckaert T, De Langhe SP, Thannickal VJ. Developmental pathways in the pathogenesis of lung fibrosis. Mol Aspects of Med. 2019;65:56–69.
Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5(7):e11803.
Doerner AM, Zuraw BL. TGF-β 1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1β but not abrogated by corticosteroids. Respir Res. 2009;10(1):1–15.
Dong J, Porter DW, Batteli LA, Wolfarth MG, Richardson DL, Ma Q. Pathologic and molecular profiling of rapid-onset fibrosis and inflammation induced by multi-walled carbon nanotubes. Arch Toxicol. 2015;89(4):621–33.
Feng L, Yang X, Liang S, Xu Q, Miller MR, Duan J, et al. Silica nanoparticles trigger the vascular endothelial dysfunction and prethrombotic state via miR-451 directly regulating the IL6R signaling pathway. Part Fibre Toxicol. 2019;16(1):16.
Fujita Y, Kosaka N, Araya J, Kuwano K, Ochiya T. Extracellular vesicles in lung microenvironment and pathogenesis. Trends in Mol Med. 2015a;21(9):533–42.
Fujita Y, Araya J, Ito S, Kobayashi K, Kosaka N, Yoshioka Y, et al. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J Extracell Vesicles. 2015b;4:28388.
Graham JR, Williams CMM, Yang Z. MicroRNA-27b targets gremlin 1 to modulate fibrotic responses in pulmonary cells. J Cell Biochem. 2014;115(9):1539–48.
Guiot J, et al. Exosomal miRNAs in lung diseases: from biologic function to therapeutic targets. J Clin Med. 2019;8(9):1345.
Hu C, Meiners S, Lukas C, Stathopoulos GT, Chen J. Role of exosomal microRNAs in lung cancer biology and clinical applications. Cell Prolif. 2020;53(6):e12828.
Itoigawa Y, Harada N, Harada S, Katsura Y, Makino F, Ito J, et al. TWEAK enhances TGF-β 1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1β but not abrogated by corticosteroids. Respir Res. 2015;16(1):48.
Iqbal MA, Arora S, Prakasam G, Calin GA, Syed MA. MicroRNA in lung cancer: role, mechanisms, pathways and therapeutic relevance. Mol Aspects Med. 2019;70:3–20.
Jeong MH, Kim HR, Park YJ, Chung KH. Akt and Notch pathways mediate polyhexamethylene guanidine phosphate-induced epithelial-mesenchymal transition via ZEB2. Toxicol Appl Pharmacol. 2019;380:114691.
Kim HR, Shin DY, Chung KH. A review of current studies on cellular and molecular mechanisms underlying pulmonary fibrosis induced by chemicals. Environ Health Toxicol. 2018a;33(3):e2018014.
Kim MS, Kim SH, Jeon D, Kim HY, Lee K. Changes in expression of cytokines in polyhexamethylene guanidine-induced lung fibrosis in mice: comparison of bleomycin-induced lung fibrosis. Toxicology. 2018b;393:185–92.
Kulshreshtha A, Ahmad T, Agrawal A, Ghosh B. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J Allergy Clin Immunol. 2013;131(4):1194–203.
Lee SJ, Park JH, Lee JY, Jeong YJ, Song JA, et al. Establishment of a mouse model for pulmonary inflammation and fibrosis by intratracheal instillation of polyhexamethyleneguanidine phosphate. J Toxicol Pathol. 2016;29(2):95–102.
Leung CC, Yu ITS, Chen W. Silicosis. The Lancet. 2012;379(9830):2008–18.
Li H, Zhao X, Shan H, Liang H. MicroRNAs in idiopathic pulmonary fibrosis: involvement in pathogenesis and potential use in diagnosis and therapeutics. Acta Pharm Sin b. 2016;6(6):531–9.
Miyaki S, Lotz MK. Extracellular vesicles in cartilage homeostasis and osteoarthritis. Curr Opin Rheumatol. 2018;30(1):129–35.
Njock MS, Guiot J, Henket MA, Nivelles O, Thiry M, Dequiedt F, et al. Sputum exosomes: promising biomarkers for idiopathic pulmonary fibrosis. Thorax. 2018;74(3):309–12.
Orgeur M, Martens M, Leonte G, Nassari S, Bonnin MA, Börno ST, et al. Genome-wide strategies identify downstream target genes of chick connective tissue-associated transcription factors. Development. 2018;145(7):dev161208.
Peng R, Sridhar S, Tyagi G, Phillips JE, Garrido R, Harris P, et al. Bleomycin induces molecular changes directly relevant to idiopathic pulmonary fibrosis: a model for “active” disease. PLoS ONE. 2013;8(4):e59348.
Pigati L, Yaddanapudi SCS, Iyengar R, Kim DJ, Hearn SA, Danforth D, et al. Selective release of MicroRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;5(10):e13515.
Rankin SA, Gallas AL, Neto A, Gómez-Skarmeta JL, Zorn AM. Suppression of Bmp4 signaling by the zinc-finger repressors Osr1 and Osr2 is required for Wnt/β-catenin-mediated lung specification in xenopus. Development. 2012;139(16):3010–20.
Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, Tendera M, et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies. Leukemia. 2012;26(6):1166–73.
Shin DY, Jeong MH, Bang IJ, Kim HR, Chung KH. MicroRNA regulatory networks reflective of polyhexamethylene guanidine phosphate-induced fibrosis in A549 human alveolar adenocarcinoma cells. Toxicol Lett. 2018;287:49–58.
Song JA, Park HJ, Yang MJ, Jung KJ, Yang HS, Song CW, Lee K. Polyhexamethyleneguanidine phosphate induces severe lung inflammation, fibrosis, and thymic atrophy. Food Chem Toxicol. 2014;69:267–75.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750.
Toyohara T, Mae SI, Sueta SI, Inoue T, Yamagishi Y, Kawamoto T, et al. Cell therapy using human induced pluripotent stem cell-derived renal progenitors ameliorates acute kidney injury in mice. Stem Cells Transl Med. 2015;4(9):980–92.
Vallecillo-García P, Orgeur M, Vom Hofe-Schneider S, Stumm J, Kappert V, Ibrahim DM, Börno ST, et al. Odd skipped-related 1 identifies a population of embryonic fibro-adipogenic progenitors regulating myogenesis during limb development. Nat Commun. 2017;8(1):1218.
Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684–707.
Zhang D, Lee H, Wang X, Groot M, Sharma L, Cruz CSD, Jin Y. A potential role of microvesicle-containing miR-223/142 in lung inflammation. Thorax. 2019;74(9):865–74.
Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2016;312(1):L110–21.
Zhang D, Lee H, Wang X, Rai A, Groot M, Jin Y. Exosome-mediated small RNA delivery: a novel therapeutic approach for inflammatory lung responses. Mol Ther. 2018;26(9):2119–30.
Zhong L, Liao G, Wang X, Li L, Zhang J, Chen Y, et al. Mesenchymal stem cells–microvesicle-miR-451a ameliorate early diabetic kidney injury by negative regulation of P15 and P19. Exp Biol Med. 2018;243(15–16):1233–42.
Funding
This work was supported by the Global Ph.D. Fellowship Program (NRF-2016H1A2A1908513) and Basic Science Research Program (NRF-2017R1D1A1B03036438) funded by the Ministry of Education, and the Korea Environmental Industry and Technology Institute (grant number 2018002490005).
Author information
Authors and Affiliations
Contributions
Mi Ho Jeong designed and performed the experiments, and wrote the manuscript; Yong Joo Park analyzed the data; and Ha Ryong Kim, Hyung Sik Kim, and Kyu Hyuck Chung conceived the design and idea of the study.
Corresponding authors
Ethics declarations
Research ethics approval
All animal experiments were approved by the Sungkyunkwan University Animal Care Committee (SKKUIACUC2019-07–09-2), and were conducted in accordance with the guidelines of the National Institutes of Health.
Consent to participate
All participates are consent to participate.
Consent to publication
All participates are consent for publication of this manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• PHMG-p, a biocide, decreases EV miR-451a significantly in conditioned media of lung epithelial cells.
• Decreased EV miR-451a upregulated its target, OSR1 in recipient cells, resulting in epithelial-mesenchymal transition.
• In in vivo, decreased EV miR-451a of BALF from PHMG-p-exposed mice contributes the upregulation of OSR1 and pulmonary fibrogenesis.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jeong, M.H., Kim, H.R., Park, Y.J. et al. Reprogrammed lung epithelial cells by decrease of miR-451a in extracellular vesicles contribute to aggravation of pulmonary fibrosis. Cell Biol Toxicol 38, 725–740 (2022). https://doi.org/10.1007/s10565-021-09626-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-021-09626-9