Abstract
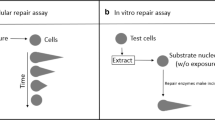
DNA repair is one of the important determinants of susceptibility to cancer. It is therefore useful to be able to measure DNA repair capacity in samples from population studies. Our aim was, first, to develop a simple comet-based in vitro assay for nucleotide excision repair (NER), similar to that already in use for base excision repair (BER), and then to apply these in vitro assays to lymphocyte samples collected on several occasions from healthy subjects, to gain an impression of the degree of intra- and inter-individual variability. The in vitro assay consists of an incubation of lymphocyte extract with substrate nucleoid DNA from cells pretreated with specific damaging agent; either photosensitiser plus light to induce 8-oxoguanine, for BER, or short wavelength ultraviolet light irradiation for NER. In the new NER assay, which requires magnesium but not adenosine triphosphate, there was significant accumulation of UV-dependent incisions during a 30-min incubation of extract with DNA. We found significant correlations between individual repair rates from samples taken on different occasions; i.e. individuals have a characteristic repair capacity. There was also significant variation between individuals, to the extent of about fourfold for BER and tenfold for NER. There was no correlation between BER and NER rates. The BER and NER assays are simple to perform and can provide valuable information in molecular epidemiological studies in which DNA instability is an endpoint.



Similar content being viewed by others
Abbreviations
- BER:
-
base excision repair
- BPDE:
-
benzo(a)pyrene diolepoxide
- FPG:
-
formamidopyrimidine DNA glycosylase
- NER:
-
nucleotide excision repair
References
Bentham G, Wolfreys AM, Liu Y, Cortopassi G, Green MHL, Arlett CF, et al. Frequencies of hprt- mutations and bcl-2 translocations in circulating human lymphocytes are correlated with United Kingdom sunlight records. Mutagenesis 1999;14:527–32.
Bonassi S, Abbondandolo A, Camurri L, Pr LD, de Rerrari M, Degrassi F, et al. Are chromosome aberrations in circulating lymphocytes predictive of future cancer onset in humans? Cancer Genet Cytogenet 1998;79:133–5.
Bonassi S, Znaor A, Ceppi M, Lando C, Chang WP, Holland N, et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis 2007;28:625–31.
Butkiewicz D, Popanda O, Risch A, Edler L, Dienemann H, Schulz V, et al. Association between the Risk for Lung Adenocarcinoma and a (−4) G-to-A Polymorphism in the XPA Gene. Cancer Epidem Biomark Prev 2004;13:2242–6.
Chen SK, Hsieh WA, Tsai MH, Chen CC, Hong AI, Wei YH, et al. Age-associated decrease of oxidative repair enzymes, human 8-oxoguanine DNA glycosylases (hOgg1), in human aging. J Radiat Res 2003;44:31–5.
Collins AR. The comet assay for DNA damage and repair. Molec Biotech 2004;26:249–61.
Collins AR, Dusinska M, Horvathova E, Munro E, Savio M, Stetina R. Inter-individual differences in DNA base excision repair activity measured in vitro with the comet assay. Mutagenesis 2001;16:297–301.
Dusinska M, Barancokova M, Kazimirova A, Harrington V, Volkovova K, et al. Does occupational exposure to mineral fibres cause DNA or chromosome damage? Mut Res 2004;553:103–10.
Dusinska M, Dzupinkova Z, Wsolova L, Harrington V, Collins AR. Possible involvement of XPA in repair of oxidative DNA damage deduced from analysis of damage, repair and genotype in a human population study. Mutagenesis 2006;2105–11.
Elliott RM, Astley S, Southon S, Archer DB. Measurement of cellular repair activities for oxidative DNA damage. Free Rad Biol Med 2000;28:1438–46.
Figueroa JD, Malats N, Real FX, Silverman D, Kogevinas M, Chanock S, et al. Genetic variation in the base excision repair pathway and bladder cancer risk. Hum Genet 2007;121:233–42.
Hagmar L, Bonassi S, Stromberg U, Brogger A, Knudsen LE, Norppa H, et al. Chromosomal aberrations in lymphocytes predict human cancer: a report from the European Study Group on Cytogenetic Biomarkers and Health (ESCH). Cancer Res 1998;58:4117–21.
Hess MT, Gunz D, Luneva N, Geacintov NE, Naegeli H. Base pair conformation-dependent excision of benzo[a]pyrene diol epoxide-guanine adducts by human nucleotide excision repair enzymes. Mol Cell Biol 1997;17:7069–76.
Hung RJ, Brennan P, Canzian F, Szeszenia-Dabrowska N, Zaridze D, Lissowska J, et al. Large-scale investigation of base excision repair genetic polymorphisms and lung cancer risk in a multicenter study. J Natl Cancer Inst 2005;97:567–76.
Ishida T, Takashima R, Fukayama M, Hamada C, Hippo Y, Fujii T, et al. New DNA polymorphisms of human MMH/OGG1 gene: Prevalence of one polymorphism among lung-adenocarcinoma patients in Japanese. Int J Cancer 1999;80:18–21.
Janssen K, Schlink K, Götte W, Hippler B, Kaina B, Oesch F. DNA repair activity of 8-oxoguanine DNA glycosylase 1 (OGG1) in human lymphocytes is not dependent on genetic polymorphism Ser326/Cys326. Mutat Res 2001;486:207–16.
Langie SAS, Knaapen AM, Brauers KJJ, van Berlo D, van Schooten F-J, Godschalk RWL. Development and validation of a modified comet assay to phenotypically assess nucleotide excision repair. Mutagenesis 2006;21:153–8.
Møller P, Wallin H, Holst E, Knudsen LE. Sunlight-induced DNA damage in human mononuclear cells. FASEB J 2002;16:45–53.
Östling O, Johanson KJ. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun 1984;123:291–8.
Paz-Elizur T, Elinger D, Leitner-Dagan Y, Blumenstein S, Krupsky M, Berrebi A, et al. Development of an enzymatic DNA repair assay for molecular epidemiology studies: Distribution of OGG activity in healthy individuals. DNA Repair 2007;6:45–60.
Reardon JT, Bessho T, Kung HC, Bolton PH, Sancar A. In vitro repair of oxidative DNA damage by human nucleotide excision repair system: possible explanation for neurodegeneration in Xeroderma pigmentosum patients. Proc Natl Acad Sci USA 1997;94:9463–8.
Redaelli A, Magrassi R, Bonassi S, Abbondandolo A, Frosina G. AP endonuclease activity in humans: development of a simple assay and analysis of ten normal individuals. Terat Carcin Mutagen 1998;18:17–26.
Roldán-Arjona T, Wei Y-F, Carter KC, Klungland A, Anselmino C, Wang R-P, et al. Molecular cloning and functional expression of a human cDNA encoding the antimutator enzyme 8-hydroxyguanine-DNA glycosylase. Proc Natl Acad Sci USA 1997;94:8016–20.
Sauvaigo S, Guerniou V, Rapin D, Gasparutto D, Caillat S, Favier A. An oligonucleotide microarray for the monitoring of repair enzyme activity toward different DNA base damage. Analyt Biochem 2004;333:182–92, Oct 1.
Sugasawa K, Ng JMY, Masutani C, Iwai S, van der Spek PJ, Eker APM, et al. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Molec Cell 1998;2:223–32.
Vodicka P, Kumar R, Stetina R, Sanyal S, Soucek P, Haufroid V, et al. Genetic polymorphisms in DNA repair genes and possible links with DNA repair rates, chromosomal aberrations and single-strand breaks in DNA. Carcinogenesis 2004;25:757–63.
Vodicka P, Stetina R, Polakova V, Tulupova E, Naccarati A, Vodickova L, et al. Association of DNA repair polymorphisms with DNA repair functional outcomes in healthy human subjects. Carcinogenesis 2007;28:657–64.
Vogel U, Moller P, Dragsted L, Loft S, Pedersen A, Sandstrom B. Inter-individual variation, seasonal variation and close correlation of OGG1 and ERCC1 mRNA levels in full blood from healthy volunteers. Carcinogenesis 2002;23:1505–9.
Wu XF, Zhao H, Wei QY, Amos CI, Zhang K, Guo ZZ, et al. XPA polymorphism associated with reduced lung cancer risk and a modulating effect on nucleotide excision repair capacity. Carcinogenesis 2003;24:505–9.
Acknowledgments
We are grateful for help with the statistical analysis from Ladislava Wsólová. We acknowledge the support of ZESPRI International Ltd, EC contract LSHB-CT-2006–037575 (COMICS), FOOD-CT-2005–016320 (NewGeneris), and the Throne Holst Fund. Isabel Gaivão was supported by FCT (Portugal), and Anita Piasek by the EC Erasmus programme. Ro 19–8022 was a gift from F. Hoffmann-La Roche.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaivão, I., Piasek, A., Brevik, A. et al. Comet assay-based methods for measuring DNA repair in vitro; estimates of inter- and intra-individual variation. Cell Biol Toxicol 25, 45–52 (2009). https://doi.org/10.1007/s10565-007-9047-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-007-9047-5