Abstract
A highly efficient nitrogen-doped carbon sheet-like material (NCS) is developed via simple pyrolysis of β-cyclodextrin and urea at different temperatures. The effect of the pyrolysis temperature (500–800 °C) on the nitrogen species present in the catalysts is thoroughly studied. These nitrogen species formed by the pyrolysis are proven to be the active sites for the chemical conversion. These catalysts containing a significant amount of nitrogen with a high degree of defects display excellent catalytic activity towards the reduction of nitro-compounds. The effect of solvents, reaction time, and temperature in nitrobenzene reduction reaction is also studied. This protocol can be easily utilized industrially due to its several excellent features such as short reaction time, use of green solvent, column chromatography free, and applicable to the gram scale. Besides, after the reaction, the NCS catalyst can be easily recovered and reused up to five runs without significant loss on its catalytic activity.
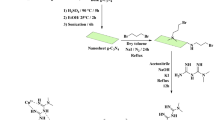
Graphical Abstract
A facile and environmentally friendly method for the synthesis of metal-free NCS and their application in nitro reduction is reported.










Similar content being viewed by others
References
Vogt PF, Gerulis JJ (2000) Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH, Weinheim, Germany
Roose P, Eller K, Henkes E, Rossbacher R, Hoke H (2000) Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH, Weinheim, Germany
Eunsuk K, Han S, Moon BK (2013) Catal Commun 45:25–29
Gupta VK, Atar N, Yola ML, Ustundag Z, Uzun L (2014) Water Res 48:210–217
Khan FA, Dash J, Sudheer Ch, Gupta RK (2003) Tetra Lett 44:7783–7787
Rai G, Jeong JM, Lee YS, Hyung WK, Lee DS, Chung JK, Myung CL (2005) Tetra Lett 46:3987–3990
Leng F, Gerber IC, Lecante P, Moldovan S, Girleanu M, Axet MR, Serp P (2016) ACS Catal 6:6018–6024
Tan X, Zhang Z, Xiao Z, Xu Q, Liang C, Wang X (2012) Catal Lett 142:788–793
Lagrost C, Preda L, Volanschi E, Hapiot PJ (2005) Electroanal Chem 585:1–7
Magdalene RM, Leelamani EG, Gowda NMN (2004) J Mol Catal A Chem 223:17–20
Sorribes I, Liu L, Corma A (2017) ACS Catal 7:2698–2708
Tian M, Cui X, Yuan M, Yang J, Ma J, Dong Z (2017) Green Chem 19:1548–1554
Torres CC, Jimenez VA, Campos CH, Alderete JB, Dinamarca R, Bustamente TM, Pawelec B (2018) Mol Catal 447:21–27
Uberman PM, Garcia CS, Rodriguez JR, Martin SE (2017) Green Chem 19:739–748
Ovoshchnikov DS, Donoeva BG, Williamson BE, Golovko VB (2014) Catal Sci Technol 4:752–757
Orlandi M, Tosi F, Bonsignore M, Benaglia M (2015) Org Lett 17:3941–3943
Lu H, Geng Z, Li J, Zou D, Wu Y, Wu Y (2016) Org Lett 18:2774–2776
Dreyer DR, Jia HP, Bielawski CW (2010) Angew Chem Int Ed 49:6813–6816
Su C, Acik M, Takai K, Lu J, Hao SJ, Zheng Y, Wu P, Bao Q, Enoki T, Chabal YJ, Loh KP (2012) Nat Commun 3:1298
Kazaoui S, Minami N, Jacquemin R, Kataura H, Achiba Y (1999) Phys Rev B 19:62–65
Chen W (2010) Doped nanomaterials and nanodevices. University of Texas at Arlington, USA
Thombal PR, Thombal RS, Han SS (2020) RSC Adv 10:474–481
Thombal RS, Jadhav VH (2015) Appl Catal A Gen 499:213–216
Gao L, Ying D, Shen T, Zheng Y, Cai J, Wang D, Zhang L (2020) ACS Sustainable Chem Eng 8:10881–10891
Shen W, Fan W (2013) J Mater Chem A 1:999–1013
Thombal PR, Han SS (2018) Biofuel Res J 5:886–893
Thombal PR, Thombal RS, Han SS (2021) Renew Sust Energ Rev 135:110218
Jiang H, Gu J, Zheng X, Liu M, Qiu X, Wang L, Li W, Chen Z, Ji X, Li J (2019) Energy Environ Sci 12:322–333
Jiang MH, Cai D, Tan N (2017) Int J Electrochem Sci 12:7154–7165
Chu H, Shao C, Qiu S, Zou Y, Xiang C, Xu F, Sun L (2018) Inorg Chem Front 5:225–232
Hu L, Peng F, Xia D, He H, He C, Fang Z, Yang J, Tian S, Sharma VK, Shu D (2018) ACS Sustain Chem Eng 6:17391–17401
Liang Q, Ye L, Huang Z, Xu Q, Bai Y, Kang F, Yang Q (2014) Nanoscale 6:13831–13837
Yuan W, Feng Y, Xie A, Zhang X, Huang F, Li S, Zhang X, Shen Y (2016) Nanoscale 8:8704–8711
Gao L, Ma J, Li S, Liu D, Xu D, Cai J, Chen L, Xie J, Zhang L (2019) Nanoscale 11:12626–12636
Liao C, Liu B, Chi Q, Zhang Z (2018) ACS Appl Mater Inter 10:44421–44429
Fujita S, Watanabe H, Katagiri A, Yoshida H, Arai M (2014) J Mol Catal A Chem 393:257–262
Xiong W, Wang Z, He S, Hao F, Yang Y, Lv Y, Zhang W, Liu P, Ha L (2020) Appl Catal 260:118105
Acknowledgements
This work was financially supported by the National Research Foundation of Korea (NRF) [grant numbers 2020R1A2C1012586, 2019R1I1A3A01062440, 2020R1A6A3A01100150]. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A1A03044512).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thombal, P.R., Rao, K.M., Zo, S. et al. Efficient Metal-Free Catalytic Reduction of Nitro to Amine Over Carbon Sheets Doped with Nitrogen. Catal Lett 152, 538–546 (2022). https://doi.org/10.1007/s10562-021-03651-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03651-3