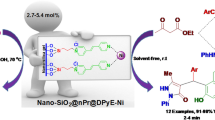
Abstract
The gel type microscopic polymer beads bearing epoxy functionalities were modified using the two-stage procedures in order to decorate their surface with the moieties of the zeroth order PAMAM type dendrimer and different heterocyclic aldehydes (2-pyridinecarboxaldehyde, 2-pyrrolidinecarboxaldehyde, furfural or 2-thiophenecarboxaldehyde). The polymeric supports provided in this manner were then used for the immobilization of copper(II) ions. The resulting materials were characterized using different instrumental techniques (optical microscopy, SEM, FTIR microscopy, DR UV–Vis, ICP-OES, and thermal analysis). They were also used as catalysts in the model A3 coupling reaction of benzaldehyde, morpholine and phenylacetylene. The best catalytic activity was found for the polymeric catalyst bearing 2-pyridinecarboxaldehyde moieties. It turned out to be effective in the A3 coupling reactions included different benzaldehyde, alkyne, and secondary amine derivatives, as well. It could also be recycled several times without a significant decrease in its activity in the model A3 coupling reaction.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Functional polymeric materials have found numerous practical applications. For instance, they are applied as polymeric sorbents for chromatography [1, 2], ion-exchangers in water treatment [3], scavenging resins for the fast purification of organic products [4], and supports for the immobilization of specific reagents and catalysts [5]. The polymers decorated with functional groups are also used in different biological applications, e.g. in bioimaging and cancer therapy [6]), and in materials science [7, 8].
Various heterogeneous polymerization techniques can be used for the synthesis of polymers with the suitable functionality. For instance, dispersion, seed, and emulsion polymerization techniques are useful from the point of view of preparation of chromatographic sorbents [1] and drug delivery systems [9]. Suspension polymerization techniques seem to be interesting particularly in the case of preparation of polymer beads for sorptive, synthetic and catalytic applications [4].
Multicomponent reactions (MCRs), i.e. the reactions included three or more reactants, are attractive as simple methods of preparation of organic compounds with the great molecular diversity. They can be useful as effective synthetic protocols for obtaining the new libraries of compounds with the potential biological activity [10,11,12,13,14,15,16], natural products [10, 14,15,16,17], agrochemicals [10, 18], substances for the electronics [19, 20], and also unique polymeric materials [21,22,23]. MCRs are characterized by higher atom economy and require less efforts comparing with the laborious step‐by‐step procedures of organic synthesis. Hence, the simplicity of MCRs can be especially attractive for the pharmaceutical industry.
Although, many multicomponent transformations are known more than hundred years (e.g. Mannich, Strecker, Ugi, Passerini and Petasis borono-Mannich reactions), the modern variants of the classic MCRs and also the new MCRs are still developed [10, 12, 24,25,26,27,28,29]. A3 coupling reactions, i.e. the reactions included alkynes, amines, and aldehydes, first discovered in late 1990s, are one of such examples [30,31,32,33,34,35]. Propargylamines yielded in the A3 couplings are important intermediates for the synthesis of numerous biologically active heterocyclic compounds, e.g. γ-lactams [36], pyrroles [37], and 2-aminoimidazoles/2-iminoimidazoles [38]. For instance, it has been found that the compounds based on propargylamines showed the activity in the treatment of Parkinson's and Alzheimer's diseases [39,40,41].
To perform the A3 coupling effectively, the selective and active catalysts are required. Numerous catalytic systems have been explored in the A3 coupling till now [30,31,32, 42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. Among them copper, silver and gold catalysts seem to play the most vital role in promoting these transformations [30, 32, 44,45,46]. Though the homogeneous version of A3 couplings shows the high efficiency, the difficulties in catalyst reusability and product purification cause that, currently, the various heterogeneous catalysts, including immobilized metal complexes [30, 34, 47, 48], coordination compounds [49, 50] and nanocatalysts [30, 32, 51,52,53,54,55] are examined intensively. The heterogeneous catalysts allow to limit polluting the final products with toxic metal species when the proper supports are selected. Hence, various multidentate ligands are designed and immobilized onto solid supports to bond strongly catalytic species on both inorganic (e.g. mesoporous silica, zeolites, and magnetic particles), organic (polymers), and hybrid (inorganic–organic) materials. To perform the heterogenization of catalytically active species both synthetic (e.g. polyacrylonitrile fibers, polystyrene resins) [56,57,58] and natural organic polymers (e.g. chitosan) might be used [59, 60].
It is well known that copper ions can promote various C-N coupling reactions, and their catalytic activity can be improved by coordinating to different multidentate ligands [34, 47, 61, 62]. Thus, it was expected that the immobilization of copper(II) ions on the polymeric supports decorated with the ligating systems composed of a polyamidoamine (PAMAM) type dendrimer and heterocyclic aldehyde moieties might provide the novel effective heterogeneous catalysts for A3 coupling reactions. Herein, we report on the synthesis of such catalytic systems based on a polymer gel with epoxy functionalities and their catalytic activity in the model A3 coupling reactions of aromatic aldehydes, secondary amines, and alkynes.
2 Experimental
2.1 General Remarks
All chemicals used in this work were purchased from Aldrich, Merck or Fluka, and used as received, unless otherwise stated. The epoxy functionalized, low-crosslinked microscopic polymer beads (GMA resin, 1.40 mmol epoxy groups per g, 75–150 μm fraction), applied as a matrix for the preparation of polymer supported copper catalysts, was synthesized according to the procedure described previously [63] using the mixture of glycidyl methacrylate (GMA, 20 mol%), styrene (S, 77 mol%) and diethylene glycol dimethacrylate (DEGDMA, 3 mol%). The zeroth order PAMAM dendrimer based on tris(2-aminoethyl)amine, methyl acrylate and ethylenediamine (EDA) was prepared according to the procedure described previously [64].
The loading of copper ions on the polymer supports was assessed using a Horiba Jobin Yvon Optima 2 ICP-OES spectrometer (λ = 223.008 nm). Samples for the ICP analysis were prepared by mineralizing the beads of the catalysts in concentrated nitric acid (≥ 69%, for trace analysis) under microwave conditions using a Plazmatronika microwave digestion system. FTIR spectra were recorded in transmission mode using a single bead method [65]. Polymer beads taken for the FTIR analyses were flattened using a micro-compression diamond cell. A Thermo Scientific Nicolet iN10MX microscope equipped with a DLaTGS detector was used to obtain appropriate spectra in the range 4000–400 cm−1. 64 or 128 scans were used to obtain the spectral resolution of 0.4 cm–1. DR UV–Vis spectra were recorded using a Jasco V-670 spectrophotometer equipped with a Jasco ISN-723 UV–Vis NIR 60 mm integrating sphere and BaSO4 as a standard. SEM measurements were performed using a VEGA3 TESCAN scanning electron microscope equipped with an energy-dispersive (EDS) detector. A Mettler Toledo (Toledo 822e) instrument was applied to conduct TGA analyses. Chromatographic analyses were performed using GC-FID or GC–MS methods. Two gas chromatographs (Agilent 7890 models) equipped with autosamplers and capillary columns HP-5 or HP-5 + MS, respectively, were applied. 1H-NMR and 13C-NMR spectra were recorded in chloroform-d using a 500 MHz Bruker Avance spectrometer.
2.2 Preparation of Polymer Supported Copper Catalysts
2.2.1 Preparation of Polymeric Supports with Dendritic Systems
To obtain the polymeric beads with dendritic functionalities (resins 1), GMA resin (2 g) was first reacted with the zeroth order PAMAM dendrimer (1.2 eq) in the medium of DMF-MeOH (3:1 v/v). The reaction was performed according to the procedure described previously [66]. The GMA-PAMAM resin (1, 0.5 g) provided in this manner was next charged to a PP syringe type reactor equipped with a cotton filter, a Luer valve and a rubber septum with a needle and swelled in 2 mL of CH2Cl2. A solution of 1.2 eq. aldehyde (2-pyridinecarboxaldehyde, PA; pyrrole-2-carboxaldehyde, PyA; 2-thiophenecarboxaldehyde, TA; or furfural, FA) in 1 mL MeOH was then added to the swollen polymeric beads. The reactor was sealed with a septum and placed on a vibrating shaker. The reaction mixtures were shaken at ambient temperature for 48 h. After that, the modified polymer beads were filtered off and rinsed several times with CH2Cl2–MeOH mixture (1:1 v/v) to remove the residuals of unreacted aldehydes. The final products (2) bearing the amine-amide-imine dendritic systems immobilized on polymeric beads were dried at 40 °C under reduced pressure.
2.2.2 Preparation of Polymer Supported Copper Catalysts
To provide polymer supported copper catalysts 3, resins 2 (0.4 g) were charged to 10 mL glass vials and swelled preliminarily in 3 mL methylene chloride. After adding copper(II) acetate solutions in 3 mL methanol (1.0 eq) to the swollen polymeric beads, the vials were sealed and placed on a vibrating shaker. The reaction mixtures were then shaken at ambient temperature for 24 h. to perform the immobilization of Cu(II) ions. The final polymeric materials were filtered off, washed several times with the mixture of solvents to remove weakly bonded Cu(II) ions, and, finally, dried at 40 °C under reduced pressure.
2.3 Catalytic Tests
Aldehyde (1 mmol), amine (1.1 mmol), phenylacetylene (1.2 mmol), polymer supported catalysts 3 (1 mol.%) and a solvent (1 mL) were charged to 5 mL vials equipped in Teflon‐coated cross‐shaped magnetic stir bars. After sealing the vials, they were placed on a StarFish® Multi‐Experiment Work Station equipped with a stirring hotplate. The reaction mixtures were gently stirred at 90 °C for the time indicated. The progress of reactions was followed by gas chromatography (GC).
The final reaction mixtures was diluted with ethyl acetate (5 mL) and the catalyst was recovered by filtration. The resulting organic solutions were concentrated under reduced pressure. The crude products of A3 coupling reaction were purified by column chromatography on silica gel using the mixtures of hexane and ethyl acetate as eluents. The structure of final products was confirmed by 1H-NMR, 13C-NMR, FT‐IR and MS spectra (see ESI).
The recycling experiments were performed in the similar manner, at 100 °C in 1,4‐dioxane. The beads of recovered catalyst 3a were washed with THF and dried under reduced pressure before their reuse.
3 Results and Discussion
3.1 Catalyst Preparation and Characterization
To obtain the new heterogeneous supports with dendritic functionalities, the epoxy functionalized polymer resin (GMA resin) in the form of microscopic beads was applied as a starting matrix. The resin was first reacted with PAMAM, similarly as in Ref. [66], to obtain resin 1 and the latter was then applied to the synthesis of a series of polymeric supports 2a–d with amine-amide-imine dendritic functionalities (Scheme 1). Heterocyclic aldehydes such as 2-pyridinecarboxaldehyde, pyrrole-2-carboxaldehyde, furfural or 2-thiophenecarboxaldehyde were used, respectively, at the second stage of the modification. The condensation of NH2 groups of immobilized PAMAM moieties with heterocyclic aldehydes was performed at room temperature in the medium of CH2Cl2–MeOH mixture (1:1 v/v). Methylene chloride delivered advantageous conditions to swell the hydrophobic polymeric matrix and activate the functional groups within spherical beads, while methanol facilitated performing the reaction of condensation under mild conditions. Finally, the beads of resins 2 swollen in CH2Cl2 were treated with a solution of copper(II) acetate in MeOH at room temperature to obtain a series of polymer supported copper(II) complexes 3a–d.
Each stage of polymer bead modification was followed by visual changes in color of the resulting beads. The starting beads were white. The reaction with PAMAM resulted in obtaining yellowish beads. As a result of the reaction of resin 1 with aldehydes, the color of beads changed into yellow. Finally, when resins 2 were contacted with the solution of Cu(CH3COO)2, the beads in green color were provided.
To gain the proof of a course of chemical modifications, the FTIR spectra of polymeric beads were recorded after each stage of transformation. Comparing the spectra of GMA resin and 1 (Fig. 1), a disappearance of absorption bands related to epoxy ring stretches (at 910 and 850 cm−1), and a strong increase in absorption intensity in the range of 3650–2450 cm−1 with maximum at 3300 cm−1 related to the stretching vibrations of NH2 (amine), N–H (amine and amide) and O–H (alcohol) groups were found. The last findings pointed out on strong mutual hydrogen bond interactions of NH2, NH and OH groups [67]. Furthermore, a series of strong bands in the range of 1660–1400 cm−1 were also observed for 1. They might be related to the vibrations of amide groups. All findings proved the successful transformation of epoxy groups under the influence of NH2 groups of PAMAM.
When the FTIR spectra of 2 were compared to the spectrum of 1, further changes could be observed. In detail, a well-defined band with the maximum absorption at 3290 cm−1 in the range characteristic of N–H and OH stretching vibrations, on the one side, and a series of well separated bands with diversified intensity in the absorption range related to C=N stretching vibration of imines and amide vibrations (1680–1500 cm−1), on the other side, were found for 2. The very strong band observed at about 1660 cm−1 is probably a consequence of overlapping the bands related to the stretching vibrations of C=O (amide) and C=N (imine) groups. The findings seemed to prove successful performing the condensation of the NH2 groups of PAMAM and the C=O groups of aldehydes. Finally, the sorption of copper(II) ions on 2 resulted in further noticeable changes in the range of 1660–1500 cm−1. For instance, the blue shifts of the absorption bands from 1658 to 1662 cm−1 and from 1547 to 1562 cm−1 were found comparing the spectra of 2 and 3. The findings seemed to prove occurring the complexation interactions between Cu(II) ions and the donor centers of dendritic ligands immobilized on the polymeric support. The strong absorption band found at 1565 cm−1 in the spectrum of 3a might be related to the vibrations of acetate ions [67]. The ions came from Cu(CH3COO)2 used as a catalyst precursor.
To gain the deeper insight regarding the course of multi-stage chemical transformation of polymeric beads, the DR UV–Vis spectra of the resulting beads were also recorded and compared (Fig. 2). It was found that the beads of GMA resin and 1 absorbed only in the UV range. The band with λmax at 266 found for the GMA resin could be related to the π–π* transitions in styrene aromatic rings and methacrylic ester moieties and that with λmax at 288 found in the spectrum of 1 seemed to prove the presence of immobilized PAMAM moieties [68]. The immobilization of heterocyclic aldehyde moieties on 1 provided the imine systems with C=N bonds conjugated with heterocycle rings. Thus, the new absorption bands observed for 2 in the visible range of UV–Vis spectrum (e.g. with λmax at 411 nm for 2a) could be related to the π–π* transitions in conjugated systems. Finally, the new absorption bands found in the spectra of 3, e.g. with λmax at 658 nm for 3a (3b—650; 3c—636; 3d —750 nm), could be attributed to the d–d transitions of Cu(II) ions in the pseudo-octahedral environment [67]. Since copper(II) acetate in the solid state absorbs at 880 nm [69], the observed blue shifts of π–π* transitions bands seemed to prove engaging the imine systems in coordinating Cu(II) ions.
To evaluate quantitatively the particular stages of the modification, the additional elemental analyses were performed for the resulting beads. Based on the values of nitrogen percentages, the loadings of PAMAM moieties and NH2 groups being able to react with heterocyclic aldehydes at the second stage were assessed. The loading of PAMAM amounted to about 1.3 mmol/g (5.7 wt.% N was found for 1). Further increases in the values of nitrogen percentages found for 2a and 2b compared to 1, on the one side, and decreases in the values for 2c and 2d, on the other side, were the additional proof of occurring the condensation between the NH2 groups of PAMAM and the C=O groups of heterocyclic aldehydes. The elemental analysis performed for 3 showed that the loadings of copper ions on polymer beads, being a consequence of the third stage of polymeric bead modification, amounted to 0.82–0.99 mmol Cu (5.19–6.32 wt% Cu). The highest loading was found in the case of 3b and the lowest for 3c (Table 1).
Comparing the SEM images recorded for GMA resin and 3a (Fig. 3), it was concluded that the multi stage modification of GMA resin did not change the morphology of polymer beads. The similar, nonporous, gel-type morphology of the beads was observed in both cases.
The thermogravimetric analysis performed for 3a revealed only about 6% weight loss after heating the beads from 25 to 200 °C (Fig. 4). It could be related to the desorption of physically absorbed solvents. The weight loss of the beads related to thermal degradation intensified only at temperature above 200 °C (Fig. 4) and achieved maximal rate at 405 °C. The findings have meant that the synthesized polymer supported complexes are thermal resistant enough to be applied to the studies of their catalytic activity under temperature conditions used commonly for A3 coupling reactions (i.e. at 90–110 °C).
3.2 Catalytic Tests
The catalytic activity of 3 was examined in the model A3 coupling reaction of benzaldehyde (BA), morpholine (MPh) and phenylacetylene (PhA) (Table 2). The reaction conditions similar to the ones described in Ref. [69] were applied. The catalysts were used in amount of 1%-mol Cu in relation to BA and the reactants in the molar proportion of 1:1.1:1.2 (BA:MPh:PhA). The relatively high excess of PhA was applied to compensate its loss in the side homocoupling reaction leading to 1,4‐diphenylbutadiyne (DPBD). It is highly probable that this reaction might accompany the A3 coupling in the presence of copper catalysts [70].
Four organic solvents differing in polarity: diglyme, 1,4-dioxane, ethylene glycol, and toluene, were first examined as a reaction medium in the presence of 3a. The time dependences of BA conversion obtained for this series of experiments allowed to conclude that ethylene glycol (Fig. 5, black line) which is a polar, protic solvent with a high dielectric constant (ε = 31.69) turned out to be the least useful medium for conducting the A3 coupling. Only about 42% BA was converted in this solvent to propargylamine within 9 h. However, using ethylene glycol as a solvent, a very few amount of DPBD (~ 0.5 wt%) has been formed after 24 h., as well (Table 2, Entry 2). The highest conversion of BA to propargylamine (~ 87%) was noted for the experiment performed in 1,4-dioxane (Fig. 5, blue line), i.e. in a non-polar, aprotic solvent with a low dielectric constant (ε = 2,21 [71]). Using this solvent, the yield of DPBD found in the crude propargylamine after 24 h. was still on the relatively low level (Table 2, Entry 3). The similar profiles of BA conversion were found for the catalytic experiments conducted in diglyme (an aprotic solvent with a medium value of dielectric constant, ε = 5.8) or toluene (a nonpolar solvent, ε = 2.38) (Fig. 5, orange and green lines, respectively), although, the former compared to the latter facilitated the formation of DPBD (Table 2, Entries 4 and 5).
To compare the catalytic activity of the developed polymer supported complexes, 1,4-dioxane was selected as an optimal reaction medium. It was found that complex 3a bearing the moieties of 2-pyridinecarboxaldehyde coupled with PAMAM turned out to be clearly more active than 3b, 3c and 3d, i.e. the complexes with pyrrole-2-carboxaldehyde, furan-2-carboxaldehyde and thiophene-2-carboxaldehyde moieties, respectively (Fig. 6; Table 3). Probably it is a consequence of much stronger donor ability of N atom in pyridine ring compared to the heteroatoms in pyrrole, furan and thiophene moieties. Among the developed catalysts, as was expected, catalyst 3d having S-donor ligands showed the lowest activity under the applied reaction conditions (Fig. 6), although in the presence of this catalyst the yield of DPBD was lower than observed for 3a (Table 2, Entry 3). The values of BA conversion found for 3b and 3c after 9 h. were on the similar level. However, the latter yielded somewhat more DPBD (Table 2, Entry 7). Despite the differences in catalytic activity, all the examined polymer supported copper complexes allowed to obtain the relatively high yields of propargylamine within 24 h. (Table 2).
A control experiment without any catalyst was also performed, however, as the results of GC analysis showed, there were no A3 coupling or PhA homocoupling products in the final reaction mixture obtained after its heating at 90 °C for 24 h. Although, about 5% conversion of BA was noted after that time. The last finding might be the result of the equilibrium reaction between BA and secondary amine resulting in the formation of secondary imine ion. The last are an intermediate in the A3 coupling reaction. However, the further transformation of imine ions to the appropriate propargylamine, being a result of the nucleophilic attack of activated phenylacetylene molecules on the carbon atom in C=N+ groups according to the commonly accepted A3 coupling mechanism [72], could not occur without a catalyst. The lack of any catalyst made the homocoupling of phenylacetylene molecules to diacetylene impossible, as well.
The activity of 3a was also examined in the reactions included substituted benzaldehydes (six examples) and two other cyclic secondary amines and substituted phenylacetylenes. In all cases, the appropriate propargylamines were obtained with good or very good yields (Table 3). Comparing the obtained results it was concluded that the exchange of morpholine (Entry 1) to piperidine (Entry 8) or pyrrolidine (Entry 9) resulted in shortening the reaction time with phenylacetylene and benzaldehyde dramatically. The suitable A3 coupling reactions were completed within about 3 h. The findings were in agreement with increasing the basicity of amines. Moreover, it was also found that the yields of the propargylamines obtained from substituted benzaldehydes in the reactions with morpholine and phenylacetylene (Entries 2–5) were generally lower than the yield obtained after the same time for the non-substituted derivative (Entry 1). Both electron donating (CH3O; Entry 2) and electron acceptor withdrawing (Cl; Entries 4–6) groups present in the aromatic ring of benzaldehyde influenced rather disadvantageously on the reactivity of benzaldehydes. The particularly negative effect on the reactivity seemed to have the presence of Cl atom at position 3 of benzaldehyde (Entry 4 and 6). The occupied positions 2 or 4 influenced much less negatively. The reactivity of 2,6-dichlobenzaldehyde was comparable to that observed for the non-substituted benzaldehyde (Entry 7). A slight improvement in the yield of the resulting propargylamine was observed only for the reaction with 4-bromobenzaldehyde (Entry 3). Finally, it was also found that the exchange of phenylacetylene to 4-methyl- or 4-methoxyphenylacetylene was related to decreasing the yields of the resulting propargylamines. The findings pointed out on a disadvantageous effect of EDGs on the reactivity of phenylacetylenes. This regularity was observed for the reactions including both morpholine (Entries 11, 13) and piperidine (Entries 10, 12). However, in the case of the reaction with morpholine the observed differences in the reactivity of acetylenes were clearly less than for piperidine. Moreover, although the reactivity of 4-Me- and 4-MeO-substituted phenylacetylenes in the reaction with morpholine was comparable, the following sequence of reactivity PhA > 4-MeO > 4-Me was observed for piperidine.
Catalyst 3a was recycled in the model reaction of benzaldehyde, phenylacetylene and morpholine. There was not observed a dramatic decrease in its activity under applied reaction conditions (Fig. 7) even during the fifth use. The final conversion of benzaldehyde after 5 h. decreased from 98% for the fresh catalyst to 90% for the fifth reaction run.
SEM analysis of the recovered catalyst showed that the physical structure of polymer beads left nearly unchangeable (Fig. 3c). The finding pointed out on the good enough mechanical resistance of the polymeric support under the applied reaction conditions. Only few mechanical damages on the rather smooth surface of a single polymer bead could be found.
The FTIR spectrum recorded for the recovered catalyst revealed a decrease in absorption in the range characteristic of acetate ions (Fig. 1). This finding indicated that acetate ions present in the fresh catalyst were removed from the coordination sphere of copper(II) ions under reaction conditions, probably, as a consequence of coordinative interactions with the reagents and solvent molecules.
An additional hot filtration test was also conducted for catalyst 3a in the reaction of benzaldehyde, morpholine, and phenylacetylene (Fig. 8). The catalyst was filtered off from the reaction mixture after 3 h. The reaction was then continued with the filtrate. The resulting kinetic data indicated that the reaction followed mainly a heterogeneous pathway. Some progress of the reaction which could be observed after the next 6 h. pointed out that the part of the immobilized copper(II) ions had been leached to the reaction mixture under the applied reaction conditions. Using ICP-OES analysis, it was found about 10% leaching of Cu(II) ions after the first run. The observed leaching of Cu(II) from the polymeric catalyst has been probably responsible for gradual decreasing in activity found for the recovered catalyst.
Comparing the activity of catalyst 3a with the activity of other polymer supported copper catalysts examined previously in the A3 coupling reactions [47, 73, 74] it can be concluded that the catalysts presented herein seem to show the relatively high activity.
4 Conclusion
The gel type microscopic polymer beads obtained by suspension polymerization from the mixture of easily accessible monomers (glycidyl methacrylate (20 mol%), styrene (77 mol%) and divinylbenzene (3 mol%) and decorated by using the simple procedures of chemical post-modification with the dendritic moieties bearing the relatively easily accessible 0th order PAMAM dendrimer and heterocyclic aldehyde (PA, PyA, TA or FA) moieties turned out to be valuable supports for the preparation of heterogeneous catalysts based on low-cost copper(II) acetate. The catalysts obtained in that manner were applied successfully in the A3 coupling reactions included various aldehyde, alkyne and secondary amine derivatives under the relatively mild reaction conditions. The best catalyst (3a), bearing PA moieties, delivered the proper alkylamines with the yield of 63–100%. It showed good recyclability (at least 5 times; 90% yield in the 5th run) in the model reaction of benzaldehyde, phenylacetylene and morpholine, as well.
References
Sýkora D, Řezanka P, Záruba K, Král V (2019) J Sep Sci 42:89
Azzouz A, Kailasa SK, Lee SS, Rascón AJ, Ballesteros E, Zhang M, Kim K-H (2018) Trends Anal Chem 108:347
Villasenõr J, Ríos A (2018) Environ Chem Lett 16:11
Tulla-Puche J, Albericio F (2008) The power of functional resins in organic synthesis. Wiley-VCH, Weinheim
Buchmeiser MR (2003) Polymeric materials in organic synthesis and catalysis. Wiley-VCH, Weinheim
Zhao W, Li A, Zhang A, Zheng Y, Liu J (2018) ChemMedChem 13:2134
Gokmen MT, Du Prez FE (2012) Prog Polym Sci 37:365
Mittal V (2011) Advanced polymer nanoparticles, Synthesis and Surface Modifications (CRC Press. Taylor&Francis Group, Boca Raton
Kim D, Shin K, Kwon SG, Hyeon T (2018) Adv Mater 30:1802309
Zarganes-Tzitzikas T, Chandgude AL, Dömling A (2015) Chem Record 15:981
Taylor AP, Robinson RP, Fobian YM, Blakemore DC, Jones LH, Fadeyi O (2016) Org Biomol Chem 14:6611
Filho JFA, Lemos BC, de Souza AS, Pinheiro S, Greco SJ (2017) Tetrahedron 73:6977
Abelraheem EMM, Shaabani S, Dömling A (2018) Drug Discov Today 29:11
Reguera L, Rivera DG (2019) Chem Rev 119:9836
Reguera L, Attorresi CI, Ramírez JA, Beilstein RDG (2019) J Org Chem 15:1236
Slobbe P, Ruijter E, Orru RVA (2012) MedChemCommun 3:1189
Schaper K, Müller TJJ (2018) Top Curr Chem 376:38
Lamberth C (2013) Pest Manag Sci 69:1106
Puthumana SSE, Damodaran B (2018) ChemistrySelect 3:2951
Zani L, Dessì A, Franchi D, Calamante M, Reginato G, Mordini A (2019) Coord Chem Rev 392:177
Jiang X, Feng Ch, Lu G, Huang X (2015) Sci China Chem 58:1695
Wu H, Gou Y, Wang J, Tao L (2018) Macromol Rapid Commun 39:1800064
Sebati W, Ray SS (2018) Catalysts 8:492
Zhu EJ, Wang Q, Wang MX (2015) Multicomponent reactions in organic synthesis. Wiley-VCH Verlag GmbH, Weinheim
Herrera ERP, Marques-Lopez E (2015) Multicomponent reactions. Concepts and applications for design and synthesis. Wiley, New York
Afshari R, Shaabani A (2018) ACS Comb Sci 20:499
Wu P, Nielsen TE (2018) Drug Discov Today Technol 29:27
Giustiniano M, Moni L, Sangaletti L, Pelliccia S, Basso A, Novellino E, Tron GC (2018) Synthesis 50:3549
Yi J, Badir SO, Alam R, Molander GA (2019) Org Lett 21:4853
Yoo WJ, Zhao L, Li CJ (2011) Aldrichchimica Acta 44(2):43
Peshkov VA, Pereshivko OP, Van der Eycken EV (2012) Chem Soc Rev 41:3790
Nasrollahzadeh M, Sajjadi M, Ghorbannezhad F, Sajadi SM (2018) Chem Rec 18:1
Mo J-N, Su J, Zhao J (2019) Molecules 24:1216
Jesin I, Nandi GCH (2019) Europ J Org Chem 14:2704
Rokade BV, Barker J, Guiry PJ (2019) Chem Soc Rev 48:4766
Shu CH, Liu MQ, Wang SS, Li L, Ye LWJ (2013) Org Chem 78:3292
Ye TY, Selvaraju M, Sun CHM (2017) Org Lett 19:3103
Jia J-H, Yu Ch, Xu M, Ma J-W, Jin H-W (2015) Synthesis 47:3473
Bolea I, Gella A, Unzeta M (2013) J Neural Transm 120:893
Zindo FT, Barber QR, Joubert J, Bergh JJ, Petzer JP, Malan SF (2014) Europ J Med Chem 80:122
Baranyi M, Porceddu PF, Gölöncsér F, Kulcsár SZ, Otrokocsi L, Kittel Á, Pinna A, Frau L, Huleatt PB, Khoo ML, Chai CHLL, Dunkel P, Mátyus P, Morelli M, Sperlágh B (2016) Mol Neurodegener 11:6
Patel SB, Vasava DV (2018) ChemistrySelect 3:471
Veisi H, Mohammadi L, Hemmati S, Tamoradi T, Mohammadi P (2019) ACS Omega 4:13991
Patel SB, Vasava DV (2020) Nano-Struct Nano-Objects 21:100416
Gholinejad M, Bonyasi R, Najera C, Saadati F, Bahrami M, Dasvarz N (2018) ChemPlusChem 83:431
Gholinejad M, Saadati F, Shaybanizadeh S, Pullithadathic B (2016) RSC Adv 6:4983
Islam MMD, Roy AS, Islam SKM (2016) Catal Lett 146:1128
Loukopoulos E, Kallitsakis M, Tsoureas N, Abdul-Sada A, Chilton NF, Lykakis IN, Kostakis GE (2017) Inorg Chem 56:4898
Li P, Regati S, Huang HC, Arman HD, Chen BL, Zhao JCG (2015) Chin Chem Lett 26:6
Liu L, Tai X, Zhou X, Xin Ch, Yan Y (2017) Sci Rep 7:12709
Gholinejad M, Afrasi M, Najera C (2019) Appl Organomet Chem 33:e4760
Patel SB, Vasava DV (2020) ChemCatChem 12:631
Terra JCS, Moores A, Moura FCC (2019) ACS Sustain Chem Eng 7:8696
Ebrahimiasl S, Behmagham F, Abdolmohammadi S, Kojabad RN, Vessally E (2019) Curr Org Chem 23:2489
Gholinejad M, Zareh F, Najera C (2018) Appl Organometal Chem 32:e4454
Li P, Liu Y, Wang L, Xiao J, Tao M (2018) Adv Synth Catal 360:1673
Hu Q, Shi X-L, Chen Y, Wang F, Weng Y, Duan P (2019) J Ind Eng Chem 69:387
Shi X-L, Sun B, Chen Y, Hu Q, Li P, Meng Y, Duan P (2019) J Catal 372:321
Shaabani A, Shadi M, Mohammadian R, Javanbakht S, Nazeri MT, Bahri F (2019) Appl Organomet Chem 33:e5074
Kaur P, Kumar B, Kumar V, Kumar R (2018) Tetrahedron Lett 59:1986
Monnier F, Taillefer M (2009) Angew Chem Int Ed 48:6954
Sambiagio C, Marsden SP, Blacker AJ, McGowan PC (2014) Chem Soc Rev 43:3525
Bukowska A, Bukowski W, Noworól J (2007) J Appl Polym Sci 106:3800
Tang MX, Redemann CT, Szoka FC Jr (1996) Biocon Chem 7:703
Yan B, Kumaravel G, Anjaria H, Wu A, Petter RC, Jewell CHF Jr, Wareing JR (1995) J Org Chem 60:5736
Bukowska A, Bukowski W, Bester K, Flaga S (2015) RSC Adv 5:49036
Pretsch E, Bühlmann P, Badertscher M (2009) Structure determination of organic compounds. Tables of spectral data. Springer, Berlin
Pande S, Crooks RM (2011) Langmuir 27:9609
Bukowska A, Bukowski W, Bester K, Hus K (2017) Appl Organomet Chem 31:e3847
Marquez C, Cirujano FG, Smolders S, Van Goethem C, Vankelecom I, De Vos D, De Baerdemaeker T (2019) Dalton Trans 48:3946
Reichardt Ch (2003) Solvents and solvent effects in organic chemistry. Wiley-VCH, Weinheim
Saha TK, Das R (2018) ChemistrySelect 3:147
Kodicherla B, Perumgani PC, Mandapati MR (2014) Appl Organomet Chem 28:756
Yan S, Pan S, Osako T, Uozumi Y (2019) ACS Sust Chem Eng 7:9097
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bukowska, A., Bester, K., Pytel, M. et al. Polymer Beads Decorated with Dendritic Systems as Supports for A3 Coupling Catalysts. Catal Lett 151, 422–434 (2021). https://doi.org/10.1007/s10562-020-03301-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03301-0