Abstract
Reduced graphene oxide supported CuO–CeO2 nanospheres (CuO–CeO2/rGO) were prepared by a facile hydrothermal method. The chemically bonded CuO–CeO2/rGO composites effectively enhanced their activity in the CO preferential oxidation (CO-PROX), while moderate rGO content (7 mg) exhibited an improvement on the catalytic activity. The prepared composite with 14 wt% rGO showed the highest catalytic activity, exceeding that of pure CuO–CeO2 and mechanically mixed CuO–CeO2 and rGO, respectively. The enhancement in the CO-PROX activity was attributed to the synergetic effect between rGO and CuO–CeO2 nanospheres. Besides supported rGO layers as excellent electron transporters, CuO–CeO2 nanospheres in turn act as the skeleton to brace two-dimensional structure to prevent from further curling, crumpling and aggregation of rGO. In the presence of capping agents during synthesis, limited catalytic activities of CuO–CeO2/rGO was observed due to the restrained interfacial charge transfer between CuO–CeO2 and rGO layers. This work, focusing on effective CO-PROX through a chemically bonded interface between CuO–CeO2 and rGO, can provide a new insight for design of new heterogeneous catalysts.
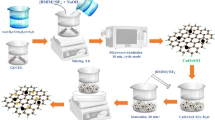
Graphical Abstract
Chemical bonded CuO–CeO2/rGO nanocomposites synthesized by a facile hydrothermal approach have shown high CO-PROX activity. Excellent charge transport between rGO layers and CuO–CeO2 nanospheres via their interfaces leads to enhanced catalytic activity for CO-PROX.











Similar content being viewed by others
References
Palo DR, Dagle RA, Holladay JD (2007) Methanol steam reforming for hydrogen production. Chem Rev 107:3992–4021
Yen H, Seo Y, Kaliaguine S et al (2012) Tailored mesostructured copper/ceria catalysts with enhanced performance for preferential oxidation of CO at low temperature. Angew Chem Int Ed 51:12032–12035
Luo MF, Ma JM, Lu JQ et al (2007) High-surface area CuO–CeO2 catalysts prepared by a surfactant-templated method for low-temperature CO oxidation. J Catal 246:52–59
Zheng XC, Zhang XL, Wang XY et al (2005) Preparation and characterization of CuO/CeO2 catalysts and their applications in low-temperature CO oxidation. Appl Catal A 295:142–149
Zhao ZK, Lin XL, Jin RH et al (2012) MOx (M = Mn, Fe, Ni or Cr) improved supported Co3O4 catalysts on ceria-zirconia nanoparticulate for CO preferential oxidation in H2-rich gases. Appl Catal B 115:53–62
Yoshida Y, Mitani Y, Itoi T et al (2012) Preferential oxidation of carbon monoxide in hydrogen using zinc oxide photocatalysts promoted and tuned by adsorbed copper ions. J Catal 287:190–202
Zhou GL, Xie HM, Gui BG et al (2012) Influence of NiO on the performance of CoO-based catalysts for the selective oxidation of CO in H2-rich gas. Catal Commun 19:42–45
Ko EY, Park ED, Lee HC et al (2007) Supported Pt-Co catalysts for selective CO oxidation in a hydrogen-rich stream. Angew Chem Int Ed 46:734–737
Kydd R, Teoh WY, Wong K et al (2009) Flame-synthesized ceria-supported copper dimers for preferential oxidation of CO. Adv Funct Mater 19:369–377
Wang F, Büchel R, Savitsky A et al (2016) In situ EPR study of the redox properties of CuO–CeO2 catalysts for preferential CO oxidation (PROX). ACS Catal 6:3520–3530
Wang WW, Du PP, Zou SH et al (2015) Highly dispersed copper oxide clusters as active species in copper-ceria catalyst for preferential oxidation of carbon monoxide. ACS Catal 5:2088–2099
Wang WW, Yu WZ, Du PP et al (2017) Crystal plane effect of ceria on supported copper oxide cluster catalyst for CO oxidation: importance of metal-support interaction. ACS Catal 7:1313–1329
Wu ZW, Zhu HQ, Qin ZF et al (2013) CO preferential oxidation in H2-rich stream over a CuO/CeO2 catalyst with high H2O and CO2 tolerance. Fuel 104:41–45
Hornés A, Hungría AB, Bera P et al (2010) Inverse CeO2/CuO catalyst as an alternative to classical direct configurations for preferential oxidation of CO in hydrogen-rich stream. J Am Chem Soc 132: 34–35.
Gao YX, Xie KM, Wang WD et al (2015) Structural features and catalytic performance in CO preferential oxidation of CuO–CeO2 supported on multi-walled carbon nanotubes. Catal Sci Technol 5:1568–1579
Atalik B, Uner D (2006) Structure sensitivity of selective CO oxidation over Pt/γ-Al2O3. J Catal 241:268–275
Fu Q, Li WX, Yao Y et al (2010) Interface-confined ferrous centers for catalytic oxidation. Science 328:1141–1144
Li X, Fang SSS, Teo J et al. (2012) Activation and deactivation of Au-Cu/SBA-15 catalyst for preferential oxidation of CO in H2-rich gas. ACS Catal 2:360–369
Biffinger JC, Uppaluri S, Sun H et al (2011) Ligand fluorination to optimize preferential oxidation of carbon monoxide by water-soluble rhodium porphyrins. ACS Catal 1:764–771
Guan H, Jian L, Lin L et al (2016) Highly active subnano Rh/Fe(OH)x catalyst for preferential oxidation of CO in H2-rich stream. Appl Catal B 184:299–308
Zhang H, Jin MS, Liu HY et al (2011) Facile synthesis of Pd-Pt alloy nanocages and their enhanced performance for preferential oxidation of CO in excess hydrogen. ACS Nano 5:8212–8222
Chen LM, Ma D, Bao XH (2007) Hydrogen treatment-induced surface reconstruction: formation of superoxide species on activated carbon over Ag/activated carbon catalysts for selective oxidation of CO in H2-rich gases. J. Phys. Chem. C 111: 2229–2234
Morfin F, Nassreddine S, Rousset JL et al (2012) Nanoalloying effect in the preferential oxidation of CO over Ir-Pd catalysts. ACS Catal 2:2161–2168
Lin J, Qiao B, Liu J et al (2012) Design of a highly active Ir/Fe(OH)x catalyst: versatile application of Pt-group metals for the preferential oxidation of carbon monoxide. Angew Chem Int Ed 51:2920–2924
Alayoglu S, Nilekar AU, Mavrikakis M et al (2008) Ru-Pt core-shell nanoparticles for preferential oxidation of carbon monoxide in hydrogen. Nat Mater 7:333–338
Qin JW, Lu JF, Cao MH et al (2010) Synthesis of porous CuO–CeO2 nanospheres with an enhanced low-temperature CO oxidation activity. Nanoscale 2:2739–2743
Gamarra D, Belver C, Fernández-García M, et al. (2007) Selective CO oxidation in excess H2 over copper-ceria catalysts: identification of active entities/species. J Am Chem Soc 129:12064–12065
Guzman J, Carrettin S, Corma A (2005) Spectroscopic evidence for the supply of reactive oxygen during CO oxidation catalyzed by gold supported on nanocrystalline CeO2. J Am Chem Soc 127:3286–3287
Bianco A, Cheng HM, Enoki T et al (2013) All in the graphene family-A recommended nomenclature for two-dimensional carbon materials. Carbon 65:1–6
Allen MJ, Tung VC, Kaner RB (2010) Honeycomb carbon: a review of graphene. Chem Rev 110:132–145
Zhou XJ, Qiao JL, Yang L et al (2014) A review of graphene-based nanostructural materials for both catalyst supports and metal-free catalysts in PEM fuel cell oxygen reduction reactions. Adv Energy Mater 4:1289–1295
Machado BF, Serp P (2012) Graphene-based materials for catalysis. Catal Sci Technol 2:54–75
Huang HJ, Yang SB, Vajtai R et al (2014) Pt-decorated 3D architectures built from graphene and graphitic carbon nitride nanosheets as efficient methanol oxidation catalysts. Adv Mater 26:5160–5165
Li YF, Zhou Z, Yu GT et al (2010) CO catalytic oxidation on iron-embedded graphene: computational quest for low-cost nanocatalysts. J Phys Chem C 114:6250–6254
Wang X, Li XY, Liu DP et al (2012) Green synthesis of Pt/CeO2/graphene hybrid nanomaterials with remarkably enhanced electrocatalytic properties. Chem Commun 48:2885–2887
Lee JS, You KH, Park CB (2012) Highly photoactive, low bandgap TiO2 nanoparticles wrapped by graphene. Adv Mater 24:1084–1088
Shang NG, Papakonstantinou P, McMullan M et al (2008) Catalyst-free efficient growth, orientation and biosensing properties of multilayer graphene nanoflake films with sharp edge planes. Adv Funct Mater 18:3506–3514
Song EH, Wen Z, Jiang Q (2011) CO catalytic oxidation on copper-embedded graphene. J Phys Chem C 115:3678–3683
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339
Wu JB, Lin ML, Cong X et al (2018) Raman spectroscopy of graphene-based materials and its applications in related devices. Chem Soc Rev 47:1822–1873
Zheng XM, Chen W, Wang G et al (2015) The Raman redshift of graphene impacted by gold nanoparticles. AIP Adv 5:1530–1534
Tivanov MS, Kolesov EA, Praneuski AG et al (2016) Significant G peak temperature shift in Raman spectra of graphene on copper. J Mater Sci 27:8879–8883
Scherrer P (1918) In: Göttinger Nachrichten Gesell, vol 2, pp. 98–100
Liu RJ, Li SW, Yu XL et al (2012) A general green strategy for fabricating metal nanoparticles/polyoxometalate/graphene tri-component nanohybrids: enhanced electrocatalytic properties. J Mater Chem 22:3319–3322
Stankovich S, Dikin DA, Piner RD et al (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Feng XF, Jiang KL, Fan SS et al (2015) Grain-boundary-dependent CO2 electroreduction activity. J. Am Chem Soc 137:4606–4609
Dongil AB, Bachiller Baeza B, Castillejos E et al (2016) The promoter effect of potassium in CuO/CeO2 systems supported on carbon nanotubes and graphene for the CO-PROX reaction. Catal Sci Technol 6:6118–6127
Ji YJ, Jin ZY, Li J et al (2017) Rambutan-like hierarchically heterostructured CeO2-CuO hollow microspheres: facile hydrothermal synthesis and applications. Nano Res 10:381–396
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21601014, 21471016 and 21271023) and the 111 Project (B07012). The authors would like to thank the Analysis & Testing Center of Beijing Institute of Technology for performing FESEM and TEM measurements.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, D., Qin, J., Wei, D. et al. Enhancing the CO Preferential Oxidation (CO-PROX) of CuO–CeO2/Reduced Graphene Oxide (rGO) by Conductive rGO-Wrapping Based on the Interfacial Charge Transfer. Catal Lett 148, 3454–3466 (2018). https://doi.org/10.1007/s10562-018-2520-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2520-3