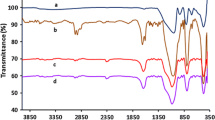
Abstract
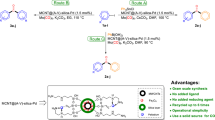
Two novel triazole-modified silica supports A and B were successfully prepared via “click” reaction of azide-functionalized SiO2 with propargyl alcohol (A) and propargyl amine (B), in which the click-triazole as an important functional entity, in addition to a molecular linker, provides capabilities of metal coordination as an excellent chelator. Treatment of the resulting click-supports with Pd(OAc)2 afforded the click-catalysts A and B, which were well characterized and evaluated in Suzuki–Miyaura coupling in terms of activity and recyclability in H2O/EtOH solvent. The catalyst A showed more reasonable results and so, was applied as a highly efficient and recyclable catalyst in the coupling reactions of various aryl halides with phenylboronic acid under phosphine-free and low Pd loading conditions.
Graphical Abstract
Two novel nano pd-catalysts were successfully prepared by immobilization of Pd(OAc)2 onto the silica supports containing the nitrogen-rich triazole chelators and were investigated in Suzuki–Miyaura coupling in aqueous solvent.












Similar content being viewed by others
References
Ricardo CL, Pintauer T (2011) Eur J Inorg Chem 1292–1301
Zeitler K, Mager I (2007) Adv Synth Catal 349:1851–1857
Gopin A, Ebner S, Attali B, Shabat D (2006) Bioconj Chem 17:1432–1440
Moses JE, Moorhouse AD (2007) Chem Soc Rev 36:1249–1262
Cai Z, Li BT, Wong EH, Weisman GR, Anderson CJ (2015) Dalton Trans 44:3945–3948
Wang C-F, Auriola S, Hirvonen J, Santos HA (2014) Curr Med Chem 21:1247–1254
Bevilacqua V, King M, Chaumontet M, Nothisen M, Gabillet S, Buisson D, Puente C, Wagner A, Taran F (2014) Angew Chem 53:5872–5876
Trindade AF, Frade RF, Maçôas EM, Graça C, Rodrigues CA, Martinho JM, Afonso CA (2014) Org Biomol Chem 12:3181–3190
Vallée MRJ, Majkut P, Krause D, Gerrits M, Hackenberger CP (2015) Chem Eur J 21:970–974
Agalave SG, Maujan SR, Pore VS (2011) Chem Asian J 6:2696–2718
Eissa AM, Khosravi E (2011) Eur Polym J 47:61–69
Guha PM, Phan H, Kinyon JS, Brotherton WS, Sreenath K, Simmons JT, Wang Z, Clark RJ, Dalal NS, Shatruk M (2012) Inorg Chem 51:3465–3477
Schulze B, Schubert US (2014) Chem Soc Rev 43:2522–2571
Pinter B, Demšar A, Urankar D, De Proft F, Košmrlj J (2011) Polyhedron 30:2368–2373
Amadio E, Bertoldini M, Scrivanti A, Chessa G, Beghetto V, Matteoli U, Bertani R, Dolmella A (2011) Inorg Chim Acta 370:388–393
Hao E, Wang Z, Jiao L, Wang S (2010) Dalton Trans 39:2660–2666
Seridi A, Wolff M, Boulay A, Saffon N, Coulais Y, Picard C, Machura B, Benoist E (2011) Inorg Chem Commun 14:238–242
Crowley JD, Bandeen PH, Hanton LR (2010) Polyhedron 29:70–83
Badèche S, Daran J-C, Ruiz J, Astruc D (2008) Inorg Chem 47:4903–4908
Wang D, Denux D, Ruiz J, Astruc D (2013) Adv Synth Catal 355:129–142
Hua C, Vuong KQ, Bhadbhade M, Messerle BA (2012) Organometallics 31:1790–1800
Veisi H, Azadbakht R, Saeidifar F, Abdi MR (2017) Catal Lett 147:976–986
Astruc D, Lu F, Aranzaes JR (2005) Angew Chem Int Ed 44:7852–7872
Veisi H, Pirhayati M, Kakanejadifard A (2017) Tetrahedron Lett 58:4269–4276
Chen X, Engle KM, Wang DH, Yu JQ (2009) Angew Chem 48:5094–5115
Lyons TW, Sanford MS (2010) Chem Rev 110:1147–1169
Veisi H, Hajimoradian Nasrabadi N, Mohammadi P (2016) Appl Organomet Chem 30:890–896
Heidari F, Hekmati M, Veisi H (2017) J Colloid Interface Sci 501:175–184
McMorn P, Hutchings GJ (2004) Chem Soc Rev 33:108–122
Campanati M, Fornasari G, Vaccari A (2003) Catal Today 77:299–314
Polshettiwar V, Len C, Fihri A (2009) Coord Chem Rev 253:2599–2626
Veisi H, Morakabati N (2015) New J Chem 39:2901–2907
Zhang G, Wang Y, Wen X, Ding C, Li Y (2012) Chem Commun 48:2979–2981
Zhang Q, Su H, Luo J, Wei Y (2013) Catal Sci Technol 3:235–243
Lv G, Mai W, Jin R, Gao L (2008) Synlett 1418–1422
He H, Gao C (2011) Curr Org Chem 15:3667–3691
Shen H, Shen C, Chen C, Wang A, Zhang P (2015) Catal Sci Technol 5:2065–2071
Jindabot S, Teerachanan K, Thongkam P, Kiatisevi S, Khamnaen T, Phiriyawirut P, Charoenchaidet S, Sooksimuang T, Kongsaeree P, Sangtrirutnugul P (2014) J Organomet Chem 750:35–40
Alonso F, Beletskaya IP, Yus M (2008) Tetrahedron 64:3047–3101
Miyaura N, Suzuki A (1995) Chem Rev 95:2457–2483
Phan NT, Van Der Sluys M, Jones CW (2006) Adv Synth Catal 348:609–679
Martin R, Buchwald SL (2008) Acc Chem Res 41:1461–1473
Wu XF, Anbarasan P, Neumann H, Beller M (2010) Angew Chem 49:9047–9050
Anctil EJ-G, Snieckus V (2002) J Organomet Chem 653:150–160
Bringmann G, Gulder T, Gulder TA, Breuning M (2010) Chem Rev 111:563–639
Hajipour AR, Hosseini SM, Mohammadsaleh F (2016) New J Chem 40:6939–6945
Hajipour A, Abolfathi P, Mohammadsaleh F (2016) RSC Adv 6:78080–78089
Hajipour AR, Abolfathi P (2016) RSC Adv 6:110622–110628
Huanga D, Zhaoa P, Astruc D (2014) Coord Chem Rev 272:145–165
Mindt TL, Struthers H, Brans L, Anguelov T, Schweinsberg C, Maes V, Tourwé D, Schibli R (2006) J Am Chem Soc 128:15096–15097
Detz RJ, Heras SA, Gelder RD, Leeuwen PWNMV., Hiemstra H, Reek JNH Maarseveen JHV (2006) Org Lett 8:3227–3230
Saleem F, Rao GK, Kumar A, Mukherjee G, Singh AK (2013) Organometallics 32:3595–3603
Yan W, Ye X, Akhmedov NG, Petersen JL, Shi X (2012) Org Lett 14:2358–2361
Talea RH, Gopulaa VB, Toradmala GK (2015) Tetrahedron Lett 56:5864–5869
Keesara S, Mandapati MR, Parvathaneni S (2015) Appl Catal A 496:58–63
Borah BJ, Borah SJ, Saikia K, Dutta DK (2014) Appl Catal A 469:350–356
Wang P, Zhu H, Liu M, Niu J, Yuan B, Li R, Ma J (2014) RSC Adv 4:28922–28927
Faria VW, Oliveira DG, Kurz MH, Gonçalves FF, Scheeren CW, Rosa GR (2014) RSC Adv 4:13446–13452
Yang J, Wang D, Liu W, Zhang X, Bian F, Yu W (2013) Green Chem 15:3429–3437
Acknowledgements
We gratefully acknowledge the funding support received for this project from the Isfahan University of Technology (IUT), IR of Iran, and Isfahan Science and Technology Town (ISTT), IR of Iran. Further financial support from the Center of Excellence in Sensor and Green Chemistry Research (IUT) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hajipour, A.R., Mohammadsaleh, F. Triazole-Functionalized Silica Supported Palladium(II) Complex: A Novel and Highly Active Heterogeneous Nano-catalyst for C–C Coupling Reactions in Aqueous Media. Catal Lett 148, 1035–1046 (2018). https://doi.org/10.1007/s10562-018-2316-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2316-5