Abstract
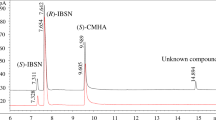
Hydroxynitrile lyases (HNLs) are increasingly finding application in the synthesis of enantiomerically pure cyanohydrins which are important intermediates in the production of pharmaceuticals and agrochemicals. Synthesis of enantiopure mandelonitrile was carried out using HNL of wild apricot (Prunus armeniaca L.) [ParsHNL] in aqueous/organic biphasic system. The optimum pH and temperature of the reaction were 4.0 and 15 °C respectively, which are important parameters to suppress the non-enzymatic catalysis. ParsHNL catalyses synthesis of (R)-mandelonitrile in methyl-tbutyl ether (MTBE)/citrate buffer biphasic system with >99% ee. Synthesis of mandelonitrile was carried out in batch reaction at 40 ml scale and finally 2.7 mmoles of (R)-mandelonitrile was recovered which corresponded to 90% molar conversion in 46 h reaction. In fed batch reaction 6.37 mmoles of (R)-mandelonitrile could be produced which corresponds to 91% molar conversion in 46 h. In both reactions, enzyme produces (R)-mandelonitrile with > 99% ee which showed enhanced selectivity as compared to aqueous reaction (96% ee) by ParsHNL. The results showed potential of ParsHNL to synthesize (R)-mandelonitrile in both, batch reaction and fed-batch reaction and can be effectively used in the synthesis of (R)-mandelonitrile.
Graphical Abstract






Similar content being viewed by others
References
Sharma M, Sharma NN, Bhalla TC (2005) Enzyme Microb Technol 37:279
Conn EE (1980) Ann Rev Plant Physiol 31:433
Gregory RJH (1999) Chem Rev 99:3649
Andexer JN, Langermann JV, Kragl U, Pohl M (2009) Trends Biotechnol 27:599
Andrea H, Clayton JR, Harvey WB (2001) Biotechnol Bioeng 74:1
Gerrits PJ, Willeman WF, Straathof AJJ, Heijnen JJ, Brussee J, Van der Gen A (2001) J Mol Catal: 15:111
Bauer M, Griengl H, Steiner W (1999) Enzyme Microb Technol 24:514
Costes D, Wehtje E, Adlercreutz P (1999) Enzyme Microb Technol 25:384
Loos WT, Geluk HW, Ruijken MMA, Kruse CG, Brussee J, Van der Gen A (1995) Biocatal Biotransform 12:255
Hickel A, Radke CJ, Blanch HW (2001) Biotechnol Bioeng 74:18
Persson M, Costes D, Wehtje E, Adlercreutz P (2002) Enzyme Microb Technol 30:916
Cascão-Pereira LG, Hickel A, Radke CJ, Blanch HW (2003) Biotechnol Bioeng 83:498
Lin G, Han S, Li Z (1999) Tetrahedron 55:3531
Techawaree U, Tamura K, Ohmiya T, Kittikun AH, Asano Y (2010) Enzyme Microb Technol 46:456
Alagöz D, Tükel SS, Yildirim D (2015) Appl Biochem Biotechnol 177:1348
Asif M, Bhalla TC (2016) Catal Lett 146:1118
Han S, Chen P, Lin G, Huang H, Li Z (2001) Tetrahedron Asym 12:843
Bradford MM (1976) Anal Biochem 72:248
Siritunga D, Arias-Garzon D, White W, Sayre RT (2004) Plant Biotech J 2:37
Alagöz D, Tükel SS, Yildirim D (2014) J Mol Catal B 101:40
Willeman WF, Hanefeld U, Straathof AJJ, Heijnen JJ (2000) Enzyme Microb Technol 27:423
Cabirol FL, Hanefeld U, Sheldon RA (2006) Adv Synth Catal 348:1645
Nanda S, Kato Y, Asano Y (2005) Tetrahedron Asym 61:10908
Acknowledgements
Authors are highly grateful to University Grants Commission (UGC), New Delhi, India for providing financial assistance to Mr. Mohammad Asif.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asif, M., Bhalla, T.C. Enantiopure Synthesis of (R)-Mandelonitrile Using Hydroxynitrile Lyase of Wild Apricot (Prunus armeniaca L.) [ParsHNL] in Aqueous/Organic Biphasic System. Catal Lett 147, 1592–1597 (2017). https://doi.org/10.1007/s10562-017-2025-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2025-5