Abstract
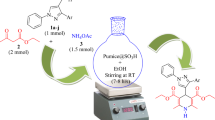
A facile and environmentally benign protocol has been developed for the one-pot three-component synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones from the cyclocondensation reaction of aldehyde, ethyl acetoacetate (β-dicarbonyl compound) and urea or thiourea using polyvinylsulfonic acid as a reusable homogeneous Brønsted acid- and metal-free organocatalyst in water and ethanol. This new protocol has the advantages of easy availability, stability, reusability and eco-friendly of the catalyst, high to excellent yields, simple experimental and work-up procedure.
Graphical Abstract



Similar content being viewed by others
References
Kappe CO (1993) Tetrahedron 49:6937
Folkers K, Johnson TB (1933) J Am Chem Soc 55:3784
Kappe CO (1997) J Org Chem 62:7201
Atwal KS, Moreland S (1991) Bioorg Med Chem Lett 1:291
Groer GJ, Dzwonczyk S, Mcmullen DM, Normadinam CS, Sleph PG, Moreland SJ (1995) J Cardiovasc Pharmacol 26:289
Kape CO (2000) Acc Chem Res 33:879
Atwal KS, Rovnyak GC, Kimball SD, Floyd DM, Moreland S, Swanson BN, Gougoutas DZ, Schewartz J, Smillie KM, Malley MF (1990) J Med Chem 33:2629
Atwal KS, Swanson BN, Unger SE, Floyd DM, Moreland S, Hedberg A (1991) J Med Chem 34:806
Klein E, DeBonis S, Thiede B, Skoufias DA, Kozielski F, Lebeau L (2007) Bioorg Med Chem 15:6474
Biginelli P (1893) Gazz Chim Ital 23:360
Hu EH, Sidler DR, Dolling UH (1998) J Org Chem 63:3454
Bose DS, Fatima L, Mereyala HB (2003) J Org Chem 68:587
Ma Y, Qian C, Wang L, Yang M (2000) J Org Chem 65:3864
Nandurkar NS, Bhanushali MJ, Bhor MD, Bhanage BM (2007) J Mol Catal A Chem 271:14
Ramalinga K, Vijyalakshmi P, Kaimal TNB (2001) Synlett 863
Paraska AS, Dewkar GK, Sudalai A (2003) Tetrahedron Lett 44:3305
Lu J, Ma H (2000) Synlett 63
Lu J, Bai Y (2002) Synthesis 466
Bhosale RS, Bhosale SV, Bhosale SV, Wang T, Zubaidha PK (2004) Tetrahedron Lett 45:9111
Liu C, Wang J, Li Y (2006) J Mol Catal A Chem 258:367
Lu J, Bai Y, Wang Z, Yang B, Ma H (2000) Tetrahedron Lett 41:9075
Reddy CV, Mahesh M, Raju PVK, Babu TR, Reddy VVN (2002) Tetrahedron Lett 43:2657
Kumar KA, Kasthuraiah M, Reddy CS, Reddy CD (2001) Tetrahedron Lett 42:7873
Yadav JS, Reddy BVS, Srinivas R, Venugopal C, Ramalingam T (2001) Synthesis 9:1341
Salitha G, Reddy GSK, Reddy KB, Yadav JS (2003) Tetrahedron Lett 44:6497
Zhang M, Li YQ (2006) Synth Commun 36:835
Liu CJ, Wang JD (2009) Molecules 14:763
Tu S, Fang F, Miao C, Jiang H, Feng Y, Shi D, Wang X (2003) Tetrahedron Lett 44:6153
Jin T, Zhang S, Li T (2002) Synth Commun 32:1847
Tu S, Fang F, Zhu S, Li T, Zhang X, Zhuang Q (2004) Synlett 537
Takale S, Parab S, Phatangare K, Pisal R, Chaskar A (2011) Catal Sci Technol 1:1128
Salehi P, Dabiri M, Zolfigol MA, Bodaghifard MA (2003) Tetrahedron Lett 44:2889
Singh K, Arora D, Singh S (2006) Tetrahedron Lett 47:4205
Rani RV, Srinias N, Kishan MR, Kulkarni SJ, Raghavan K (2003) Green Chem 3:305
Jain SL, Joseph JK, Singhal S, Sain B (2007) J Mol Catal A Chem 268:134
Bigi F, Carloni S, Frullanti B, Maggi R, Sartori G (1999) Tetrahedron Lett 40:3465
Yadav JS, Reddy BVS, Sridhar P, Reddy JSS, Nagaiah K, Lingaiah N, Saiprasd PS (2004) Eur J Org Chem 552
Reddy PN, Reddy YT, Reddy MN, Rajitha B, Crooks PA (2009) Synth Commun 39:1257
Wang L, Cai C (2008) J Heterocycl Chem 45:1771
Heravi MM, Bakhtiari L, Bamoharram FF (2006) Catal Commun 7:373
Donadoni A, Massi A (2001) Tetrahedron Lett 42:7975
Chandak HS, Lad NP, Upare PP (2009) Catal Lett 131:469
Debache A, Amimour M, Belfaitah A, Rhouati S, Carboni B (2008) Tetrahedron Lett 49:6119
Legeay JC, Eunde JJV, Bazureau JP (2005) Tetrahedron 61:12386
Peng J, Deng Y (2001) Tetrahedron Lett 42:5917
Polshettiwar V, Varma RS (2007) Tetrahedron Lett 48:7343
Esenberg H, Mohan GR (1959) J Phys Chem 63:671
Distler H (1965) Angew Chem Int Ed 4:300
Okayasu T, Saito K, Nishide H, Hearn MTW (2009) Chem Commun 4708
Mitsutani A (2002) Catal Today 73:57
Hino M, Arata K (1985) Appl Catal 18:401
Eliel EL, Fisk MT, Prosser T (1963) Org Synth 4:169
Kuma AK, Chottopadhyay TK (1987) Tetrahedron Lett 28:3713
Koster R, Linden BV, Poels E, Blick A (2001) J Catal 204:333
Rahmatpour A (2011) Monatsh Chem 142:1259
Rahmatpour A (2011) React Funct Polym 71:80
Rahmatpour A (2011) Heteroat Chem 22:51
Rahmatpour A (2011) Heteroat Chem 22:85
Rahmatpour A (2012) Monatsh Chem 143:491
Breslow DS, Kutner A (1958) J Polym Sci 27:295
Sakurada I, Sakaguchi Y, Ono T, Ueda T (1966) Die Makromol Chem 91:243
Joseph JK, Jain SL, Sain B (2006) J Mol Catal A Chem 247:99
Lin H, Zhao Q, Xu B, Wang X, Mole J (2007) J Mol Catal A Chem 268:221
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahmatpour, A. Polyvinylsulfonic Acid: An Efficient, Water-Soluble and Reusable Brønsted Acid Catalyst for the Three-Component Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones/thiones in Water and Ethanol. Catal Lett 142, 1505–1511 (2012). https://doi.org/10.1007/s10562-012-0873-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-012-0873-6