Abstract
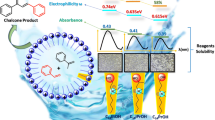
In this work, the chloromethylation reaction of aromatic compounds was performed successfully by micellar catalysis in oil/water biphasic system at high reactant loadings that exceeded the solubilization capacity of micellar solutions. The effects of cationic, nonionic and anionic surfactants on the reaction were compared. The mechanism of chloromethylation reaction and the mechanism of micellar catalysis were investigated. The results show that the micellar catalysis is an effective way to realize the chloromethylation. The chloromethylation reaction consists of electrophilic substitution reaction and nucleophilic substitution reaction. Cationic surfactants, especially those containing longer hydrophobic carbon chain, are more effective. Selectivity for mono-chloromethylation was remarkably improved and regioselectivity was found to be dependent on the nature of the surfactant. Under the optimal reaction conditions, chloromethylation of isopropylbenzene could obtain 97.5% selectivity in mono-chloromethylation and 8.28 para/ortho selectivity ratio at 89.8% conversion.
Graphical Abstract
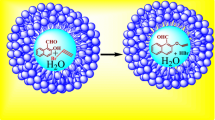
In oil/water biphasic system, surfactant micelles can catalyze the chloromethylation reaction of aromatic compounds effectively and different types of surfactants have different catalytic abilities. Good conversion and high selectivity in mono-chloromethylation were obtained.







Similar content being viewed by others
References
Abramo JG, Chapin EC (1961) J Org Chem 26:2671
Royals EE, Prasad RN (1955) J Am Chem Soc 77:1696
Wright ME, Toplikar EG, Svejda SA (1991) Macromolecules 24:5879
Huang ZT, Wang GQ, Yang LM (1995) Synth Commun 25:1109
Mcnamara CA, Dixon MJ, Bradley M (2002) Chem Rev 102:3275
Belen’kii LI, Vol’kenshtein YuB, Karmanova IB (1977) Russ Chem Rev 46:891
Pinell RP, Khune GD, Khatri NA, Manatt SL (1984) Tetrahedron Lett 25:3511
DeHaan FP, Djaputra M, Grinstaff MW, Kaufman CR, Keithly JC, Kumar A, Kuwayama MK, Macknet KD, Na J, Patel BR, Pinkerton MJ, Tidwell JH, Villahermosa RM (1997) J Org Chem 62:2694
Selva M, Trotta F, Tundo P (1991) Synthesis 11:1003
McKillop A, Madjdabadi FA, Long DA (1983) Tetrahedron Lett 24:1933
Formentin P, Garcia H (2002) Catal Lett 78:115
Wang Y, Shang ZC, Wu TX (2006) Synth Commun 36:3053
Maudling DR, Lotts KD, Robinson SA (1983) J Org Chem 48:2938
Olah GA, Beal DA, Olah JA (1976) J Org Chem 41:1627
Kachurin OL, Zaraiskii AP, Velichko LL, Zaraiskaya NA, Matvienko NM, Okhrimenko ZA (1995) Russ Chem Bull 44:1815
Kishida T, Yamauchi T, Kubotab Y, Sugi Y (2004) Green Chem 6:57
Miyagawa CC, Kupka J, Schumpe A (2005) J Mol Catal A Chem 234:9
Battal T, Siswanto C, Rathman J (1997) Langmuir 13:6053
Samant BS, Saraf YP, Bhagwat SS (2006) J Colloid Interface Sci 302:207
Blagoeva IB, Toteva MM, Quarti N, Ruasse MF (2001) J Org Chem 66:2123
Maria PD, Fontana A, Gasbarri C, Siani G (2005) Tetrahedron 61:7176
Durand A (2006) J Mol Catal A Chem 256:284
Harustiak M, Hronec M, Ilavsky J, Witek S (1998) Catal Lett 1:391
Fernandes MLM, Krieger N, Baron AM, Zamora PP, Ramos LP, Mitchell DA (2004) J Mol Catal B Enzym 30:43
Siswanto C, Rathman JF (1997) J Colloid Interface Sci 196:99
Liu QF, Lu M, Li YQ, Li J (2007) J Mol Catal A Chem 277:113
Gao BJ, Liu QF, Jiang LD (2008) Chem Eng Process 47:852
Kishida T, Yamauchi T, Komura K, Kubota Y, Sugi Y (2006) J Mol Catal A Chem 246:268
Zhu BY, Zhao ZG (1996) Fundamentals of interfacial chemistry (in Chinese). Chemical Industry Press, Beijing
Tascioglu S (1996) Tetrahedron 52:11113
Ogata Y, Okano M (1956) J Am Chem Soc 78:5423
Olah GA, Yu SH (1975) J Am Chem Soc 97:2293
Nazarov IN, Semenovsky AV (1957) Russ Chem Bull 6:225
Wang F, Liu H, Cun LF, Zhu J, Deng JG, Jiang YZ (2005) J Org Chem 70:9424
Freeman SK (1961) J Org Chem 26:212
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Q., Wei, W., Lu, M. et al. Chloromethylation of Aromatic Compounds Catalyzed by Surfactant Micelles in Oil–Water Biphasic System. Catal Lett 131, 485–493 (2009). https://doi.org/10.1007/s10562-009-9926-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-009-9926-x