Abstract
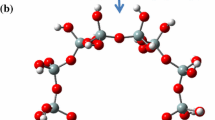
The density functional theory (DFT/B3LYP) calculations were applied to investigate the interaction of a Pt6 particle with the ZSM-5 zeolite framework. The electronic structure of the metal particle is strongly affected by the interaction with basic framework oxygens and acid sites of the zeolite support. Adsorption on basic sites (Eads = 6 kcal/mol) favors the formation of the electron enriched metal cluster. Interaction of the platinum cluster with the acid site characterized by stabilization energy of 47 kcal/mol results in oxidation of the metal particle and suppression of Brønsted acidity of the support. The hypothesis is put forward that the oxidized platinum particle can function as an active site for the alkane isomerisation on platinum supported high silica zeolites.




Similar content being viewed by others
References
Bandiera J, Naccache C, Imelik B (1978) J Chim Phys–Chim Biol 75:406
Chupin J, Gnep NS, Lacombe S, Guisnet M (2001) Appl Catal A 206:43
Blomsma E, Martens JA, Jacobs PA (1997) Stud Surf Sci Catal B 105:909
Galperin LB, Bricker JC, Holmgren JR (2003) Appl Catal, A 239:297
Sugioka M, Tochiyama C, Matsumoto Y, Sado F (1995) Stud Surf Sci Catal 94:544
Vasina TV, Masloboishchikova OV, Khelkovskaya-Sergeeva EG, Kustov LM, Houzvička JI (2001) Stud Surf Sci Catal 138:93
Arribas MA, Martinez A (2002) Appl Catal A 230:203
Noordhoek NJ, Schuring D, de Gauw FJMM, Anderson BG, de Jong AM, de Voigt MJA, van Santen RA (2002) Ind Eng Chem Res 41:1973
Kuznetsov PN (2003) J Catal 218:2
Weisz PB, Swegler EW (1957) Science 126:31
Kuhlmann A, Roessner F, Schwieger W, Gravenhorst O, Selvam T (2004) Catal Today 97:303
Ono Y (2003) Catal Today 81:3
Libuda J, Frank M, Sandell A, Andersson S, Brühwiler PA, Bäumer M, Mårtensson N, Freund H-J (1997) Surf Sci 384:106
Heemeier M, Frank M, Libuda J, Wolter K, Kuhlenbeck H, Bäumer M, Freund H-J (2000) Catal Lett 68:19
Stakheev AYu, Sachtler WMH (1991) J Chem Soc, Faraday Trans 87:3703
Blackmond DG, Goodwin JG Jr (1981) J Chem Soc Chem Commun 125
Sachtler WMH, Zhang Z (1993) Adv Catal 39:129
Vaarkamp M, Miller JT, Modica FS et al. (1993) Stud Surf Sci Catal 75:809
Zholobenko VL, Lei GD, Carvill BT et al. (1994) J Chem Soc, Faraday Trans 90:233
Weber RS, Boudart M, Gallezot P (1980) Stud Surf Sci Catal 4:415
Ramaker DE, Mojet BL, Garriga Oostenbrink MT, Miller JT, Koningsberger DC (1999) Phys Chem Chem Phys 1:2293
Hammer B, Nørskov JK (1995) Nature 376:238
Koningsberger DC, Oudenhuijzen MK, Bitter JH, Ramaker DE (2000) Topics Catal 10:167
Koningsberger DC, Ramaker DE, Miller JT, de Graaf J, Mojet BL (2001) Topics Catal 15:35
Kubička D, Kumar N, Venäläinen T, Karhu H, Kubičková I, Österholm H, Murzin YuD (2006) J Phys Chem, B 110:4937
Vayssilov GN, Gates BC, Rösch N (2003) Angew Chem Int Ed 42:1391
Vayssilov GN, Rösch N (2005) Phys Chem Chem Phys 7:4019
Treesukol P, Srisuk K, Limtrakul J, Truong TN (2005) J Phys Chem, B 109:11940
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Vosko SH, Wilk L, Nusair M (1980) Can J Phys 58:1200
Stevens WJ, Krauss M, Basch H, Jasien PG (1992) Can J Chem 70:612
Granovsky AA, PC GAMESS version 7.0, http://classic.chem.msu.su/gran/gamess/index.html
Schmidt MW, Baldridge KK, Boatz JA et al. (1993) J Comput Chem 14:1347
NBO 4.M. Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Weinhold F (1999) Theoretical Chemistry Institute, University of Wisconsin, Madison, WI
Stakheev AY, Kustov LM (1999) Appl Catal, A 188:3
Blekkan E, Pham-Huu C, Ledoux MJ, Guille J (1994) Ind Eng Chem Res 33:1657
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mikhailov, M.N., Kustov, L.M. & Kazansky, V.B. The State and Reactivity of Pt6 Particles in ZSM-5 Zeolite. Catal Lett 120, 8–13 (2008). https://doi.org/10.1007/s10562-007-9245-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-007-9245-z