Abstract
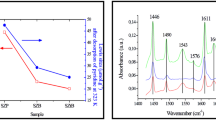
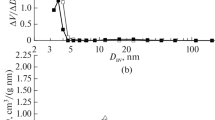
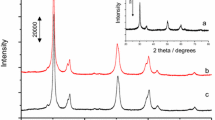
Calcination parameters, such as atmosphere, duration and catalyst bed depth have a marked influence on the catalytic and spectroscopic properties of sulfated zirconia. Sulfated zirconia calcined in nitrogen or synthetic airflow, in deep bed, exhibited comparable activity in n-butane isomerization at 373 K, which suggests that oxygen is not necessary for formation of active sites. Catalysts calcined in shallow bed are catalytically inactive. Thus, the bed depth is concluded to be crucial for the formation of active sites. The samples calcined in shallow bed possessed lower sulfate content and the S=O stretching vibration was located at lower frequency. Calcination in the presence of water vapor also led to lower catalytic activity, sulfate content, and BET area. Extended calcination reduced gradually the activity and the sulfate content, which underlines the labile property of the active sites. A new interpretation of the function of the calcination step is proposed and compared with models described in the literature.






Similar content being viewed by others
References
Song X.M., Sayari A. (1996). Catal. Rev. Sci. Eng. 38:329
Arata K. (1996). Appl. Catal. A Gen. 146:3
Cheung T.-K., Gates B.C. (1998). Top. Catal. 6:41
Adeeva V., Liu H., Xu B., Sachtler W.M.H. (1998). Top. Catal. 6:61
Fărcaşiu D., Li J.Q. (1998). Appl. Catal. A Gen. 175:1
Hong Z., Fogash K. B., Dumesic J. A. (1999). Catal. Today 51:269
Yadav G., Nair J. J. (1999). Micropor. Mesopor. Mater. 33:1
Yamaguchi T. (2001). Appl. Catal. A Gen. 222:237
K. Tanabe, M. Itoh, K. Morishige and H. Hattori (1976), in: Preparation of Catalysts, eds. B. Delmon, P. Jacobs and G. Poncelet (Elsevier, Amsterdam) p. 65.
Yamaguchi T., Tanabe K., Kung Y. C. (1986). Mater. Chem. Phys. 16:67
Sohn J.R., Kim H.W. (1989). J. Mol. Catal. 52:361
Tran M.-T., Gnep N.S., Szabo G., Guisnet M. (1998). Appl. Catal. A Gen. 171:207
Morterra C., Cerrato G., Signoretto M. (1996). Catal. Lett. 41:101
Morterra C., Cerrato G., Meligrana G., Signoretto M., Pinna F. Strukul G. (2001). Catal. Lett. 73:113
Li X., Nagaoka K., Olindo R., Lercher J.A. (2006). J. Catal. 238:39
Li X., Nagaoka K., Simon L.J., Olindo R., Lercher J.A., Hofmann A., Sauer J.A.(2005). J. Am. Chem. Soc. 127:16159
Hahn A. H. P., Jentoft R. E., Ressler T., Weinberg G., Schlögl R., Jentoft F. C. (2005). J. Catal. 236:324
Canton P., Olindo R., Pinna F., Strukul G., Riello P., Meneghetti M., Cerrato G., Morterra C., Benedetti A. (2001). Chem. Mater. 13:1634
Chokkaram S., Srinivasan R., Milburn D.R., Davis B.H. (1994). J. Colloid Interface Sci. 165:160
Srinivasan R., Keogh R. A., Milburn D. R., Davis B. H. (1995). J. Catal. 153:123
Srinivasan R., Davis B. H. (1993). J. Colloid Interface Sci. 156:400
Comelli R. A., Vera C. R., Parera J. M. (1995). J. Catal. 15:96
Chen F.R., Coudurier G., Védrine J.F. (1993). J. Catal. 143:616
Hertl W. (1989). Langmuir 5:96
Tsyganenko A.A., Filimonov V.N. (1973). J. Mol. Struct. 19:579
Yamaguchi T., Jin T., Tanabe K. (1986). J. Phys. Chem. 90:3148
Waqif M., Bachelier J., Saur O., Lavalley J. C. (1992). J. Mol. Catal. 72:127
Acknowledgments
The financial support of the Deutsche Forschungsgemeinschaft (DFG) is gratefully acknowledged. We thank Prof. Helmut Papp and Dr. Friederike Jentoft for fruitful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Nagaoka, K., Simon, L.J. et al. Influence of calcination procedure on the catalytic property of sulfated zirconia. Catal Lett 113, 34–40 (2007). https://doi.org/10.1007/s10562-006-9005-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-006-9005-5