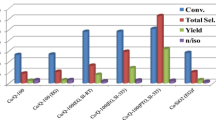
A new method for heterogenization of metal complex catalysts by precipitation of its water–soluble analogue as a Gr.2 metals (Ca, Sr or Ba) salt on porous supports has been proposed. This technique yields a highly dispersed catalyst having a significantly higher activity (TOF) for hydroformylation of olefins compared to other known heterogenized catalysts. The catalyst can be recycled with ease.
Similar content being viewed by others
References
(a) B. Cornils and W.A. Hermann, Aqueous Phase Organometallic Catalysis-Concept and Application (Wiley-VCH Weinheim, Germany 1998), p.4. (b) B. Cornils and W.A. Hermann, J. Catal. 216 (2003) 23. (c) D.J. Cole-Hamilton, Science 299 (2003) 1702.
(a) B. Cornils, J. Mol. Catal. A: Chem. 143 (1999) 1. (b) P.W.N.M. van Leeuven and C. Claver, eds. Rhodium Catalyzed Hydroformylation (Kluwer, Dordrecht 2000), p. 203.
(a) E.G. Kuntz, Chemtech (1987) 570. (b) P. Kalck and F. Monteil, Adv. Organomet. Chem. 34 (1992) 219. (c) W.A. Hermann and C.W. Kohlpaintner, Angew. Chem. Ind. Ed. Engl. 32 (1993) 1524. (d) F. Monteil and P. Kalck, J. Organomet. Chem. 177 (1994) 480. (e) F. Ungvary, Coord. Chem. Rev. 147 (1996) 547. (f) F. Joo, J. Mol. Cat. A: Chem. 116 (1997) 3. (h) B. E. Hanson, Coord. Chem. Rev. 185 (1999) 795.
(a) R.R. Hartley, Supported Metal Complexes (Reidel, Dordrechiet, 1985). (b) P.R. Roney, J. F. Roth, US Patent 3.855.307 (1974). (c) H. Hjortkjaer, S.M. Scurrell and P. Simonsen J. Mol. Cat. 12 (1981) 179.
(a) D.C. Bailey and J.H. Langer, Chem. Rev. 81(1981) 109. (b) K. Mukhopadhyay, A.B. Mandale and R.V. Chaudhari, Chem. Mater.15 (2003) 1766.
(a) R. Augustine, S. Tanielyan, S. Anderson and H. Yang, Chem. Commun.(2001)1257. (b) S. Tanielyan, R. Augustine US Patent 6025245 (1999). (c) M.J. Bark, A. Gerlach, D. Semmerji, J. Org. Chem. 65 (2000) 8933. (d) K. MuKhopadhyay and R.V. Chaudhari, J. Catal. 213 (2003) 73.
(a) A. Hu, H.L. Ngo and W. Lin J. Am. Chem. Soc. 125 (2003) 11490. (b) A. Hu, H.L. Ngo and W. Lin, Angew. Chem. Int. Ed. 42 (2003) 6000.
I.W Davies D.L HughesMatty L L Matty P.J Reider (2001) J. Am. Chem. Soc. 123 125
P Arhancet M.E Davis J.S Merola B.E Hanson (1990) J. Cat. 121 327 Occurrence Handle1:CAS:528:DyaK3cXitlOit74%3D Occurrence Handle10.1016/0021-9517(90)90241-B
See supporting information.
The 31P nmr signal for Ba3/2TPPTS and carbon supported Ba3/2TPPTS is observed at − 2.78 and 3.43δ. For C-II the δ value is 29.14 (doublet) for the coordinated P nuclei. The corresponding phosphine oxide appears in the same range as a part of the signal.
(a) Reaction conditions: HRhCO(PPh3)3: 2.18 × 10−3 kmol/m3, Rh:PPh3 = 1:6 (excess), 1-decene : 0.4224 kmol/m3, T: 1000C, pCO+H2(1:1): 4.14 MPa, Stirring speed : 1000 rpm, Solvent : Toluene, Total charge: 2.5 × 10−5m3, Time : 0.9 h. (b) [Rh(COD)Cl]2: 1.48 × 10−3 kmol/m3 (aq.), Rh:TPPTS = 1:6 (excess) 1-decene: 0.704 kmol/m3 (org.), T: 100 °C, Stirring speed : 1000 rpm, pCO+H2 (1:1): 4.14 MPa, Solvent : Toluene + Water (aqueous phase hold up = 0.4), Total charge : 2.5 × 10−5 m3, Time: 6.6 h.
M. Nowotny T. Maschmeyer Brian F.G Johnson P. Lahuerta J.M Thomas J.E Davies (2001) Angew. Chem. Int. Ed. 5 40
New Synthesis with Carbon monoxide, edited by J. Falbe (Springler-Verlag Berlien, Heidelberg, New york 1980), pp. 84–89.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pagar, N.S., Deshpande, R.M. & Chaudhari, R.V. Hydroformylation of olefins using dispersed molecular catalysts on solid supports. Catal Lett 110, 129–133 (2006). https://doi.org/10.1007/s10562-006-0095-x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10562-006-0095-x