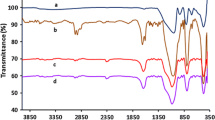
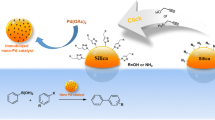
The chitosan–palladium catalyst supported on silica was prepared and used to catalyze an asymmetry transfer hydrogenation of prochiral acetophenone to alcohol with formate salts as a hydrogen donor at atmosphere. The results indicate that palladium coordinates with amino group in chitosan to form palladium–chitosan complex and its performance for an asymmetry transfer hydrogenation of acetophenone is affected greatly by the reaction temperature, hydrogen donor and solvent. For the hydrogenation of acetophenone over the palladium–chitosan–silica catalyst, the suitable solvent is ethanol and hydrogen donor is ammonium formate or sodium formate. Using ammonium formate as H-donor at atmosphere and 80 °C, an enantiomeric excess of R-1-phenylethanol reaches 68.9%.
Similar content being viewed by others
References
G.I.P. Daniëlle C.J.K. Paul L.S. Anthony (2000) J. Org. Chem. 65 3010
A. Baiker (1998) Curr. Opin. Solid State Mater. Sci. 3 86
W.K. Sang J.B. Sung H. Taeghwan (2001) Micropor. Mesopor. Mater. 523 44–45
H. Takeda K. Kadowaki (1985) Agric. Biol. Chem. 49 3151
M.Y. Yin G.L. Yuan Y.Q. Wu (1999) J. Mol. Catal. A: Chem. 147 93
J. Zhang C.G. Xia (2002) J. Mol. Catal. (China), 16 465
E. Piron M. Accomintti A. Domard (1997) Langmuir 13 1653
O. Takeshi O. Hirohito H. Shohei (1995) J. Am. Chem. Soc. 117 2675
C. Salazzo R. Halle T. Francois (2000) J. Organomet. Chem. 603 30
M. Tommo K. Yoshinori E. Kokki (1998) J. Org. Chem. 63 2378
R. Alice H. Domien T. Francois (2001) Tetra: Asy. 12 811
T. Nakano J. Ando Y. Jshii (1987) Tech. Rep. Kansai Univ. 29 69
S. Papp A. Szücs I. Dékány (2001) Appl. Clay. Sci. 19 155
A.K. Zharmagambetova E.E. Ergozhin Y.L. Sheludyakov S.G. Mukhamedzhanova I.A. Kurmanbayeva B.A. Selenova B.A. Utkelov (2001) J. Mol. Catal. A: Chem. 177 165
S. Kajagopal A.F. Spatola (1995) J. Org. Chem. 60 1347
G. Brieger T. Nestrick (1974) Chem. Rev. 74 567
R.A.W. Johnstone A.H. Wilby I.D. Entwistle (1985) Chem. Rev. 85 129
G. Cavinato L. Toniolo (1996) J. Mol. Catal A: Chem. 106 25
S. Rajagopal A.F. Spatola (1997) Appl. Catal. A: Gen. 152 69
J.B. Daniel B.T. Catherine E.C.P. Mano (1998) Tetra.: Asy. 9 2015
P.N. Rylander (1976) Catalytic Hydrogenation over Platinum Metals Academic Press New York
M.V. Rajashekharam I. Bergault P. Fouilloux D. Schweich H. Delmas R.V. Chaudhari (1999) Catal. Today 48 83
G. Cavinato L. Toniolo (1996) J. Mol. Catal. A: Chem. 106 25
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Y., Guo, Y., Lu, Q. et al. Highly selective asymmetry transfer hydrogenation of prochiral acetophenone catalyzed by palladium–chitosan on silica. Catal Lett 100, 213–217 (2005). https://doi.org/10.1007/s10562-004-3458-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10562-004-3458-1