Abstract
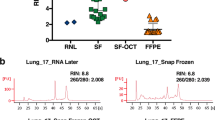
RNA degradation is a major problem in tissue banking. We explored the effect of thawing flash-frozen biospecimens on the quality and integrity of RNA for genetic testing as well as for other cancer research studies. The histological quality of the frozen tumor sections was evaluated by using hematoxylin and eosin staining. RNA extraction from 60 lung cancer tissue samples subjected to various freeze/thaw cycles was performed using the RNeasy Plus isolation kit. RNA integrity was assessed by using an Agilent bioanalyzer to obtain RNA integrity numbers (RIN). Furthermore, RNA from different groups was used for fluorescence Reverse transcription-polymerase chain reaction (RT-PCR) analysis of the echinoderm microtubule-associated protein-like 4 and anaplastic lymphoma kinase (EML4-ALK) fusion gene mutation to verify whether it can be used for research or clinical testing. Highly variable RIN values were observed among the samples, which showed no correlation with the number of freeze/thaw cycles conducted. However, after 3 freeze/thaw cycles (each thaw event lasted for 10 min), an increasing number of changes in peak intensity in RINs were observed. After 5 freeze/thaw cycles, RNA integrity decreased to approximately 35%. After 3 freeze/thaw cycles, the RNA could still be used for RT-PCR analysis of EML4-ALK fusion gene mutations; whereas those subjected to 5 freeze/thaw cycles could not. Limited (<3) freeze/thaw cycles did not adversely affect the quality of RNA extracted from tumor tissues and subsequent RT-PCR analysis. Our data could be utilized in the establishment of a standardized procedure for tissue biospecimen collection and storage.




Similar content being viewed by others
References
Aibadula A, Lu JF, Wu JS et al (2015) Establishment and maintenance of a standardized glioma tissue bank: Huanshan experience. Cell Tissue Bank 16(2):271–281
Bild AH, Yao G, Chang JT et al (2006) Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 439(7074):353–357
Chu TY, Hwang KS, Yu MH et al (2002) A research-based tumor tissue bank of gynecologic oncology: characteristics of nucleic acids extracted from normal and tumor tissues from different sites. Int J Gynecol Cancer 12:171–176
Ellis HJ, Venturini DS (2013) Demonstration of a frozen sample aliquotter to prepare plasma and serum aliquots without thawing frozen parent samples. Biopreserv Biobank 11(3):153–160
Grizzle WE, Bell WC, Sexton KC (2010) Issues in collecting, processing and storing human tissues and associated information to support biomedical research. Cancer Biobank 9(1–6):531–549
Johnsen IK, Hahner S, Briere JJ et al (2010) Evaluation of a standardized protocol for processing adrenal tumor samples: preparation for a European adrenal tumor bank. Horm Metab Res 42:93–101
Liu JG, Zhao RY, Zhang J et al (2015) ARMS for EGFR mutation analysis of cytologic and corresponding lung adenocarcinoma histologic specimens. J Cancer Res Clin Oncol 141(2):221–227
Micke P, Ohshima M, Tahmasebpoor S et al (2006) Biobanking of fresh frozen tissue: RNA is stable in nonfixed surgical specimens. Lab Invest 86:202–211
Morales PJ (2012) The importance of establishing an international network of tissue banks and regional tissue processing centers. Cell Tissue Bank 10:232–233
Ransohoff DF, Gourlay ML (2010) Sources of bias in specimens for research about molecular markers for cancer. J Clin Oncol 28:698–704
Rooney P, Eagle MJ, Kearney JN (2015) Validation of cold chain shipping enviroment for transport of allografts as part of a human tissue bank returns policy. Cell Tissue Bank 16(4):533–538
Schroeder A, Mueller O, Stocker S et al (2006) The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol 7:3
Shabihkhani M, Lucey GM, Wei B et al (2014) The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin Biochem 47(4–5):258–266
Sun HY, Sun R, Hao M et al (2016) Effect of duration of ex vivo ischemia time and storage period on RNA quality in biobanked human renal cell carcinoma. Ann Surg Oncol 23(1):297–304
Wang Y, Zheng H, Chen J et al (2015) The impact of different preservation conditions and freezing-thawing cycles on quality of RNA, DNA, and proteins in cancer tissue. Biopreserv Biobank 13(5):335–347
Yasojima K, McGeer EG, McGeer PL (2001) High stability of mRNAs postmortem and protocols for their assessment by RT-PCR. Brain Res Brain Res Protoc 8:212–218
Youlden DR, Cramb SM, Baade PD (2008) The international epidemiology of lung cancer: geographical distribution and secular trends. Thorac Oncol 3:819–831
Yu KK, Zhang J, Li X et al (2015) Establishment and management of a lung cancer biobank in Eastern China. Thorac Cancer 6:58–63
Zhang Y, Jin B, Shao M et al (2014) Monitoring of carcinoembryonic antigen levels is predictive of EGFR mutations and efficacy of EGFR-TKI in patients with lung adenocarcinoma. Tumour Biol 5:4921–4928
Acknowledgements
The Shanghai JiaoTong University “985 Project” biological sample library and the Shanghai R&D Public Service Platform Project “Common malignant tumor biobank (network) construction” supported this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Keke Yu and Jie Xing are the co-first authors.
Rights and permissions
About this article
Cite this article
Yu, K., Xing, J., Zhang, J. et al. Effect of multiple cycles of freeze–thawing on the RNA quality of lung cancer tissues. Cell Tissue Bank 18, 433–440 (2017). https://doi.org/10.1007/s10561-016-9600-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-016-9600-7