Abstract
In 1986, the National Nuclear Energy Agency (Batan) in Jakarta started the research and development for the setting up of a tissue bank (Batan Research Tissue Bank/BRTB) by preserving fresh amnion or fetal membranes by lyophilisation and then sterilising by gamma irradiation. During the period of 1990 and 2000, three more tissue banks were set up, i.e., Biomaterial Centre in Surabaya, Jamil Tissue Bank in Padang, and Sitanala Tissue Bank in Tangerang. In 1994, BRTB produced bone allografts. The banks established under the IAEA program concentrated its work on the production of amnion, bone and soft tissues allografts, as well as bone xenografts. These tissues (allografts and xenografts) were sterilised using gamma irradiation (about 90%) and the rest were sterilized by ETO and those products have been used in the treatment of patients at more than 50 hospitals in Indonesia. In 2004, those tissue banks produced 8,500 grafts and 5,000 of them were amnion grafts for eye treatment and wound dressing. All of those grafts were used for patients as well as for research. In 2006, the production increased to 9,000 grafts. Although the capacity of those banks can produce more grafts, we are facing problems on getting raw materials from suitable donors. To fulfill the demand of bone grafts we also produced bone xenografts. The impact of the IAEA program in tissue banking activities in Indonesia can be summarised as follows: to support the national program on importing substitutes for medical devices. The price of imported tissues are between US$ 50 and US$ 6,000 per graft. Local tissue bank can produce tissues with the same quality with the price for about 10–30% of the imported tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The early period
The “Indonesia 1992 Health Regulation” provides for procurement of tissues from living donors as well as from deceased donors. Since 85% of the Indonesian population is Moslem, a “Fatwa” from Moslem Religious Council (MUI) for deceased donors should be obtained. A “Fatwa for Bone, Skin and Amnion” was introduced by the MUI on June 29, 1997 permitting tissue procurement from cadaver donors.
The development of all specialty medical field in Indonesia provide the impetus for providing biological tissues for medical treatment, which cause strong motivation for the development of tissue banking in Indonesia. This was started before 1990. The National Nuclear Energy Agency (Batan) Jakarta started the research and development on the setting up of tissue bank (Batan Research Tissue Bank/BRTB) in 1986 by carrying out research on preserving fresh amnion or foetal membranes by lyophilisation and then sterilising by gamma irradiation. In 1994, BRTB started to produce bone allograft obtained from live donors.
In 1992, Dr. Soetomo General Hospital in Surabaya set up a bone bank which produced frozen bone sterilised by ethylene oxide (ETO) and in 2000 it became The Biomaterial Centre—“Dr. Soetomo” Tissue Bank and produced variable tissues sterilised by irradiation such as: fresh frozen and freeze dried bone, fresh frozen and freeze dried amniotic membrane, fresh frozen and freeze dried tendon, fresh frozen and freeze dried fascia. In 1997, a tissue bank was set up at M. Jamil Hospital in Padang and produced amnion grafts sterilised by radiation.
Before tissue bank was set up in Indonesia the orthopedic surgeons used commercial products of allograft or bone substitutes, but unfortunately, the price was too high for Indonesian people. This situation presented a challenge for orthopaedic surgeons and other scientists to develop tissue banks in Indonesia. After tissue banks were developed and followed by public campaigning, the demanding of allografts both fresh frozen and freeze dried has increased very much.
Within the 1990s, four tissue banks were founded in the state. The IAEA provided financial, technical, as well as training support for tissue bank staffs and the users of tissue bank products to consolidate these four tissue banks:
-
a)
Batan Research Tissue Bank in Jakarta.
-
b)
Biomaterial Center at Soetomo Hospital in Surabaya.
-
c)
Tissue Bank at M.D jamil Hospital in Padang.
-
d)
Tissue Bank at Sitanala Hospital in Tangerang.
The banks established under the IAEA program concentrate their work on the production of amnion, bone and soft tissues allograft, as well as bone xenografts. These tissues (allograft and xenografts) were sterilized using gamma irradiation (about 90%) and the rest were sterilised by ETO. Those products have been used in the treatment of patients at more than 50 hospitals in Indonesia. In 2004, these tissue Banks produced 8,542 grafts and 5,000 of them were amnion grafts for eye treatment, as well as for wound dressing. All of those grafts were used for patients as well as for researches. In 2006, the production increase up to 9,000 grafts. Although the capacity of those banks can produce more grafts but we are facing problems to get raw materials from suitable donors. To fulfill the demand of bone grafts we also produced bone xenografts.
In 2003, a bone bank was established in Solo, Central Java, i.e., at the Prof. Dr. Soeharso Orthopaedic Hospital (Hilmy et al. 2007; Hilmy and Paramita 2006; Ferdiansyah 2006).
Types of graft produced
-
A.
Human bone/allograft
-
I.
Freeze dried tissue
-
1.
Calvarial bone (bi-cortical)
-
2.
Costae
-
3.
Ilium (tri and bi cortical)
-
4.
Cortical strut graft (fibula and costae)
-
5.
Cortical chips
-
6.
Cancellous chips
-
7.
Bone powder
-
II.
De-mineralized and freeze dried tissues
-
1.
Cortical chips and powder
-
2.
Cancellous chips and powder
-
1.
-
III.
Soft tissues
-
3.
Tendon
-
4.
Facia
-
5.
Amnion
-
3.
-
B.
Bovine bone/xenograft
-
1.
Cancellous chips
-
2.
De-mineralized bone powder
-
3.
Eye-ball
-
4.
Strut
-
1.
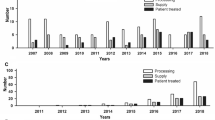
The type of allograft produced per year can be seen in Table 1 (those are products, which were produced in 2004). Increasing of products was not significant because of lack of suitable donors. In 2006, total tissues produced were about 9,000 pieces, 90% of them were sterilised by radiation and the rest by ETO mostly for frozen bone allograft. Although the needs for allograft are increasing, those tissue banks could not fulfil it based on lack of suitable donor.
Xenografts are also produced by BRTB as well as by Biomaterial Centre, and the number of grafts produced are around 1 000 graft per year.
Increasing tissue banking activities and the impact of the IAEA program
The progresses of tissue banks activities are very much influenced by the rising demand of various biological tissues, as well as implementation of quality assurance in all steps of the activities. Having obtained more than six years experiences (from 1988 to 1994), from just producing amniotic membrane and storing frozen bone; nowadays there are many kind of tissue produced such as bone, fascia, tendon and other soft tissues from selected cadaver and surgical donor. Established tissue banks perform good standard procedure of processing, preservation, and sterilisation as recommended by EATB, AATB, and IAEA. The production reached 9,000 grafts per year in 2006, with improving quality of product since we adopted the organisation and international standards, as well as good developing processing techniques learnt from several tissue banks centers. There was an impact also of the training of seven tissue bank staffs in the Regional Training Centre in Singapore; one staff in advance training in Bangkok and two staffs in North West Tissue Centre, USA. IAEA also support us by training 20 users of tissue bank products in several countries like USA.
The impact of the IAEA program in tissue banking activities in Indonesia can be summarised as follows:
-
Support the national program on import substitute for medical devices. The price of imported tissues are between US$ 50 and US$ 6,000 per graft. Local tissue bank can produce tissues with the same quality with the price for about 10–30% of the imported tissues.
-
Increasing the ability, quality and confident of human resources, such as operators of tissue banks and clinical users.
-
Confidence in producing any kind of tissue allografts since we are implementing the IAEA International Standard in Tissue Banking.
As a result of the IAEA program, at present Indonesia is applying the following IAEA documents as well as International standard such as:
-
a)
IAEA International Standard for Tissue bank.
-
b)
IAEA Code of Practice for the Radiation Sterilisation of Tissues Allografts: Requirements for Validation and Routine Control.
-
c)
ISO 11137/2006 for Validation of Radiation Sterilisation Dose.
Since a new ISO 11137 has been established in 2006, we do hope that IAEA Code will be updated and re-edited in order to be related with this new ISO.
The IAEA program supported the implementation of one of national project INS/7/003 “Radiation Sterilisation of Human Tissue Grafts” during the period 1995–2000, with a budget of US$ 255,391. Indonesia also participated in the implementation of two regional project RAS/7003 “Radiation Sterilisation of Tissue Grafts” (RCA) during the period 1988–1998, and RAS/7/008 “Quality Assurance in Radiation Sterilisation of Tissue Graft” (RCA) during the period 1997–2003, and two interregional projects INT/6/049 “Interregional Centre of Excellence in Tissue Banking” during the period 1997–2003, and INT/6/052 “Improving the Quality of Production and Uses of Radiation Sterilised Tissue Grafts” from 2002 to 2004 (Hilmy et al. 2006; Manjas et al. 2006).
The current situation of tissue banking activities
The established tissue banks in Indonesia can now produce any type of bone grafts, any kinds of de-mineralized bone grafts, soft tissue such as tendon, facia, as well as amnion grafts and any kind of bone xenografts. These four banks has now eight tissue bank operators with International certificates and more than 20 medical personnel working full or part time. Most of them were trained using several of IAEA programs, mostly in using bone, soft tissues, and amnion grafts.
The Indonesian’s authorities are ready to support the presentation of a program or a project to the IAEA, in order to consolidate tissue banking activities in a selected group of countries in Asia and the Pacific, Europe, and Latin American regions, if the governments of other countries are prepared to adopt the same position.
The tissue banks in Indonesia are now cooperating with other tissue banks in Singapore and Malaysia.
According to the future demand of tissue in the country, the established tissue banks should concentrate their activities in producing bone, soft tissues, skin, as well as amnion grafts to be used for medical treatment.
Caused by the increasing demand in tissue bank products, as stated at the annual meeting of the Indonesian Association of Tissue Bank (IATB) held in 2001, it was decided to establish two more tissue banks at South Sulewesi and Central Java province. In 2003, a tissue bank was established in Solo, Central Java.
In the opinion of Indonesian’s authorities, the following factors are limiting the increase of tissues donors and are affecting tissue banking activities in the country:
-
a)
Lack of National Coordinators established by government and interest in becoming donors.
-
b)
Problems of suitable irradiator of Co-60, such as increasing the Co-60 sources of a Gamma Cell in Padang. The Gamma Cell was obtained from IAEA 1996, but now the sources are only 3,000 Ci. Padang is located in Sumatra and about 600 miles from Jakarta. We could not help them to sterilise their products. We do hope that IAEA will support us in increasing the Co-60 sources.
-
c)
New Emerging Infectious Diseases caused by viruses such as avian influenza type H5N1, Corona virus, West Nile Virus, dengue virus which make the donor could not be screened according to IAEA standard. We do hope that IAEA will held a new project on eliminating viruses from grafts by radiation.
To overcome these limiting factors the Indonesian’s authorities are considering the adoption of the following actions.
-
a)
Establishing a National Coordinator to support obtaining suitable donor.
-
b)
Increasing production of xenografts.
-
c)
Joining IAEA new project in tissue banking.
Conclusions
It can be concluded that Indonesia still need IAEA support to fulfill the increasing demand of tissue allografts, as well as research on the effects of radiation on viruses.
References
Hilmy N, Paramita P (2006) New emerging infectious diseases caused by viruses. In: Nather A, Yusof N, Hilmy N (eds) Radiation in Tissue Banking. World Scientific, Singapore, pp 133–146
Hilmy N, Pandansari P, Ibrahim GS, Indira S, Bambang S, Sunarti R, Herawati S (2006) Use of freeze-dried irradiated amnion in ophthalmologic practices. In: Nather A, Yusof N, Hilmy N (eds) Radiation in tissue banking. World Scientific, Singapore, pp 355–364
Hilmy N, Febrida A, Basril A (2007) Experiences using IAEA code for tissue allografts. J Rad Physc Chem 76:1751–1755
Ferdiansyah (2006) Use of freeze-dried irradiated bones in orthopedic surgery in radiation. In: Nather A, Yusof N, Hilmy N (eds) Tissue banking. World Scientific, Singapore, pp 317–326
Manjas M, Tarusaraya P, Hilmy N (2006) The use of irradiated amnion grafts in wound dressing. In: Nather A, Yusof N, Hilmy N (eds) Tissue Banking. World Scientific, Singapore, pp 329–342
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hilmy, N., Manjas, M., Ferdiansyah et al. The impact of the International Atomic Energy Agency (IAEA) program on radiation and tissue banking in Indonesia. Cell Tissue Bank 10, 103–107 (2009). https://doi.org/10.1007/s10561-008-9088-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-008-9088-x