Abstract
Purpose
This study aimed to explore the effect of microRNA (miR)-145 on cardiac fibrosis in heart failure mice and its target.
Methods
Experiments were carried out in mice receiving left coronary artery ligation, transverse aortic constriction (TAC), or angiotensin (Ang) II to trigger heart failure, and in cardiac fibroblasts (CFs) with Ang II-induced fibrosis.
Results
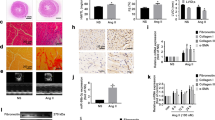
The miR-145 levels were decreased in the mice hearts of heart failure induced by myocardial infarction (MI), TAC or Ang II infusion, and in the Ang II-treated CFs. The impaired cardiac function was ameliorated by miR-145 agomiR in MI mice. The increased fibrosis and the levels of collagen I, collagen III, and transforming growth factor-beta (TGF-β) in MI mice were inhibited by miR-145 agomiR or miR-145 transgene (TG). The agomiR of miR-145 also attenuated the increases of collagen I, collagen III, and TGF-β in Ang II-treated CFs. Bioinformatics analysis and luciferase reporter assays indicated that mitogen-activated protein kinase kinase kinase 3 (MAP3K3) was a direct target gene of miR-145. MAP3K3 expression was suppressed by MiR-145 in CFs, while the MAP3K3 over-expression reversed the inhibiting effects of miR-145 agomiR on the Ang II-induced increases of collagen I, collagen III, and TGF-β in CFs.
Conclusion
These results indicated that miR-145 upregulation could improve cardiac dysfunction and cardiac fibrosis by inhibiting MAP3K3 in heart failure. Thus, upregulating miR-145 or blocking MAP3K3 can be used to treat heart failure and cardiac fibrosis.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Park GS, An MK, Yoon JH, et al. Botulinum toxin type a suppresses pro-fibrotic effects via the JNK signaling pathway in hypertrophic scar fibroblasts. Arch Dermatol Res. 2019;311:807–14.
Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5:15.
Kwak HB. Aging, exercise, and extracellular matrix in the heart. J Exerc Rehabil. 2013;9:338–47.
Flevaris P, Vaughan D. The role of plasminogen activator inhibitor Type-1 in fibrosis. Semin Thromb Hemost. 2017;43:169–77.
Parichatikanond W, Luangmonkong T, Mangmool S, Kurose H. Therapeutic targets for the treatment of cardiac fibrosis and cancer: focusing on TGF-beta signaling. Front Cardiovasc Med. 2020;7:34.
Hanna A, Humeres C, Frangogiannis NG. The role of Smad signaling cascades in cardiac fibrosis. Cell Signal. 2021;77:109826.
To KKW, Fong W, Tong CWS, Wu M, Yan W, Cho WCS. Advances in the discovery of microRNA-based anticancer therapeutics: latest tools and developments. Expert Opin Drug Discov. 2020;15:63–83.
Zhang B, Tian L, Xie J, Chen G, Wang F. Targeting miRNAs by natural products: a new way for cancer therapy. Biomed Pharmacother. 2020;130:110546.
Arcidiacono B, Chiefari E, Foryst-Ludwig A, et al. Obesity-related hypoxia via miR-128 decreases insulin-receptor expression in human and mouse adipose tissue promoting systemic insulin resistance. EBioMedicine. 2020;59:102912.
Cakmak HA, Demir M. MicroRNA and cardiovascular diseases. Balkan Med J. 2020;37:60–71.
Kura B, Szeiffova Bacova B, Kalocayova B, Sykora M, Slezak J. Oxidative stress-responsive microRNAs in heart injury. Int J Mol Sci. 2020;21:358.
Huang S, Zhang L, Song J, et al. Long noncoding RNA MALAT1 mediates cardiac fibrosis in experimental postinfarct myocardium mice model. J Cell Physiol. 2019;234:2997–3006.
Liu Y, Song JW, Lin JY, Miao R, Zhong JC. Roles of MicroRNA-122 in cardiovascular fibrosis and related diseases. Cardiovasc Toxicol. 2020;20:463–73.
Yang X, Yu T, Zhang S. MicroRNA-489 suppresses isoproterenol-induced cardiac fibrosis by downregulating histone deacetylase 2. Exp Ther Med. 2020;19:2229–35.
Zhang M, Cheng YJ, Sara JD, et al. Circulating MicroRNA-145 is associated with acute myocardial infarction and heart failure. Chin Med J. 2017;130:51–6.
Wu J, Huang Q, Li P, et al. MicroRNA-145 promotes the epithelial-mesenchymal transition in peritoneal dialysis-associated fibrosis by suppressing fibroblast growth factor 10. J Biol Chem. 2019;294:15052–67.
Liu Z, Tao B, Fan S, et al. Over-expression of microRNA-145 drives alterations in beta-adrenergic signaling and attenuates cardiac remodeling in heart failure post myocardial infarction. Aging (Albany NY). 2020;12:11603–22.
Xu Z, Sun J, Tong Q, et al. The role of ERK1/2 in the development of diabetic cardiomyopathy. Int J Mol Sci. 2016;17. https://doi.org/10.3390/ijms17122001
Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40.
Csak T, Bala S, Lippai D, et al. microRNA-122 regulates hypoxia-inducible factor-1 and vimentin in hepatocytes and correlates with fibrosis in diet-induced steatohepatitis. Liver Int. 2015;35:532–41.
Castillero E, Akashi H, Najjar M, et al. Activin type II receptor ligand signaling inhibition after experimental ischemic heart failure attenuates cardiac remodeling and prevents fibrosis. Am J Physiol Heart Circ Physiol. 2020;318:H378–H90.
Yang Z, Zhang X, Guo N, Li B, Zhao S. Substance P inhibits the collagen synthesis of rat myocardial fibroblasts induced by Ang II. Med Sci Monitor: Int Med J Exp Clin Res. 2016;22:4937–46.
Yang X, Wang Y, Yan S, et al. Effect of testosterone on the proliferation and collagen synthesis of cardiac fibroblasts induced by angiotensin II in neonatal rat. Bioengineered. 2017;8:14–20.
Wolfien M, Klatt D, Salybekov AA, et al. Hematopoietic stem-cell senescence and myocardial repair - coronary artery disease genotype/phenotype analysis of post-MI myocardial regeneration response induced by CABG/CD133+ bone marrow hematopoietic stem cell treatment in RCT PERFECT phase 3. EBioMed. 2020;57:102862.
Wojciechowska A, Braniewska A, Kozar-Kaminska K. MicroRNA in cardiovascular biology and disease. Adv Clin Exp Med. 2017;26:865–74.
Zhang J, Wei X, Zhang W, Wang F, Li Q. MiR-326 targets MDK to regulate the progression of cardiac hypertrophy through blocking JAK/STAT and MAPK signaling pathways. Eur J Pharmacol. 2020;872:172941.
Song JJ, Yang M, Liu Y, et al. MicroRNA-122 aggravates angiotensin II-mediated apoptosis and autophagy imbalance in rat aortic adventitial fibroblasts via the modulation of SIRT6-elabela-ACE2 signaling. Eur J Pharmacol. 2020;883:173374.
Dutka M, Bobinski R, Korbecki J. The relevance of microRNA in post-infarction left ventricular remodelling and heart failure. Heart Fail Rev. 2019;24:575–86.
Vegter EL, van der Meer P, de Windt LJ, Pinto YM, Voors AA. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail. 2016;18:457–68.
Sun D, Yang F. Metformin improves cardiac function in mice with heart failure after myocardial infarction by regulating mitochondrial energy metabolism. Biochem Biophys Res Commun. 2017;486:329–35.
Adapala RK, Kanugula AK, Paruchuri S, Chilian WM, Thodeti CK. TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation. Basic Res Cardiol. 2020;115:14.
Polegato BF, Minicucci MF, Azevedo PS, et al. Association between functional variables and heart failure after myocardial infarction in rats. Arq Bras Cardiol. 2016;106:105–12.
Wang K, Zhu ZF, Chi RF, et al. The NADPH oxidase inhibitor apocynin improves cardiac sympathetic nerve terminal innervation and function in heart failure. Exp Physiol. 2019;104:1638–49.
Gabisonia K, Prosdocimo G, Aquaro GD, et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature. 2019;569:418–22.
Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016;118:1021–40.
Cui S, Liu Z, Tao B, et al. miR-145 attenuates cardiac fibrosis through the AKT/GSK-3beta/beta-catenin signaling pathway by directly targeting SOX9 in fibroblasts. J Cell Biochem. 2021;122:209–21.
Zhao L, Ni X, Zhao L, et al. MiroRNA-188 acts as tumor suppressor in non-small-cell lung Cancer by targeting MAP3K3. Mol Pharm. 2018;15:1682–9.
Zhang L, Zhang Y, Wang S, et al. MiR-212-3p suppresses high-grade serous ovarian cancer progression by directly targeting MAP3K3. Am J Transl Res. 2020;12:875–88.
Lv Y, Huang Y, Xu M, et al. The miR-193a-3p-MAP3k3 signaling Axis regulates substrate topography-induced Osteogenesis of bone marrow stem cells. Adv Sci (Weinh). 2020;7:1901412.
Yu C, Liu Y, Sun L, et al. Chronic obstructive sleep apnea promotes aortic remodeling in canines through miR-145/Smad3 signaling pathway. Oncotarget. 2017;8:37705–16.
Sawant D, Klevenow E, Baeten JT, et al. Generation of transgenic mice that conditionally express microRNA miR-145. Genesis. 2020;58:e23385.
Author information
Authors and Affiliations
Contributions
Yun Liu performed the research. Jing Hu analyzed the data. Weiwei Wang wrote the paper. Qian Wang designed the research study and revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the Ethical Committee on the Experimental Animal Care and Use Committee of Nanjing Medical University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Fig. S1
The level of microRNA (miR)-145 after treating with miR-145 agomiR. (A) The level of miR-145 was increased in the hearts of mice after treating with miR-145 agomiR. (B) The level of miR-145 was increased in the cardiac fibroblasts (CFs) after treating with miR-145 agomiR. All results are expressed as mean ± standard error of the mean.*P < 0.05 versus the NC agomiR group. (PNG 588 kb)
Fig. S2
Effects of mitogen-activated protein kinase kinase kinase 3 (MAP3K3) overexpression or knockdown in cardiac fibroblasts (CFs). (A) The level of MAP3K3 was increased by MAP3K3 overexpression. (B) The level of MAP3K3 was increased by MAP3K3 knockdown. N = 6 for each group. All results are expressed as mean ± standard error of the mean.*P < 0.05 versus the Ad-GFP group. (PNG 487 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Hu, J., Wang, W. et al. MircroRNA-145 Attenuates Cardiac Fibrosis Via Regulating Mitogen-Activated Protein Kinase Kinase Kinase 3. Cardiovasc Drugs Ther 37, 655–665 (2023). https://doi.org/10.1007/s10557-021-07312-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-021-07312-w