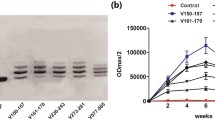
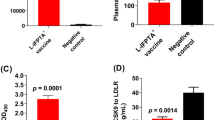
Abstract
Purpose
Our group has developed a therapeutic vaccine targeting proprotein convertase subtilisin/kexin type 9 (PCSK9), named PCSK9Qβ-003. In this study, we investigated the potential effectiveness of the PCSK9Qβ-003 vaccine on atherosclerosis.
Methods
Male ApoE−/− mice were randomly assigned to three groups: a phosphate-buffered saline (PBS) group, Qβ virus-like particles (VLP) group, and PCSK9Qβ-003 vaccine group. Mice in the PCSK9Qβ-003 group were injected with the PCSK9Qβ-003 vaccine four times (100 μg/time) over a period of 18 weeks. The effects of the vaccine on atherosclerotic plaque, cholesterol transport, inflammation and apoptosis were investigated.
Results
The PCSK9Qβ-003 vaccine obviously decreased total cholesterol and low-density lipoprotein cholesterol in ApoE−/− mice. Compared with the other groups, the PCSK9Qβ-003 vaccine significantly reduced the lesion area and promoted the stability of atherosclerotic plaque. The vaccine regulated cholesterol transport in the aorta of ApoE−/− mice by up-regulating the expression level of liver X receptor α and ATP binding cassette transporter A1. Additionally, macrophage infiltration and expression of monocyte chemoattractant protein-1 and tumor necrosis factor-α were significantly decreased in the mice administered the PCSK9Qβ-003 vaccine. The vaccine also markedly reduced apoptosis in the lesion area of the aorta in ApoE−/− mice.
Conclusions
The results demonstrated that the PCSK9Qβ-003 vaccine attenuated the progression of atherosclerosis by modulating reverse cholesterol transport and inhibiting inflammation infiltration and apoptosis, which may provide a novel therapeutic approach for atherosclerosis and greatly improve treatment compliance among patients.






Similar content being viewed by others
References
Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–26.
Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–36.
Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47.
Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–93.
Abifadel M, Varret M, Rabès JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154.
Abifadel M, Rabès JP, Devillers M, et al. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum Mutat. 2009;30:520–9.
Lopez D. PCSK9: an enigmatic protease. Biochim Biophys Acta. 2008;1781:184–91.
Zhao Z, Tuakli WY, Lagace TA, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79:514–23.
Raal FJ, Stein EA, Dufour R, et al. RUTHERFORD-2 investigators. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:331–40.
Ginsberg HN, Rader DJ, Raal FJ, et al. Efficacy and safety of Alirocumab in patients with heterozygous familial hypercholesterolemia and LDL-C of 160 mg/dl or higher. Cardiovasc Drugs Ther. 2016;30:473–83.
Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743–53.
Bachmann MF, Dyer MR. Therapeutic vaccination for chronic diseases: a new class of drugs in sight. Nat Rev Drug Discov. 2004;3:81.
Chackerian B, Remaley A. Vaccine strategies for lowering LDL by immunization against proprotein convertase subtilisin/kexin type 9. Curr Opin Lipidol. 2016;27:345–50.
Pan Y, Zhou Y, Wu H, et al. Therapeutic peptide vaccine against PCSK9. Sci Rep. 2017;7:12534.
Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309.
Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–16.
Vainio S, Ikonen E. Macrophage cholesterol transport: a critical player in foam cell formation. Ann Med. 2003;35:146–55.
Lee SD, Tontonoz P. Liver X receptors at the intersection of lipid metabolism and atherogenesis. Atherosclerosis. 2015;242:29–36.
Pascual GM, Valledor AF. Biological roles of liver X receptors in immune cells. Arch Immunol Ther Exp. 2012;60:235–49.
Oosterveer MH, Grefhorst A, Groen AK, Kuipers F. The liver X receptor: control of cellular lipid homeostasis and beyond implications for drug design. Prog Lipid Res. 2010;49:343–52.
Kolovou V, Marvaki A, Boutsikou M, et al. Effect of ATP-binding cassette transporter A1 (ABCA1) gene polymorphisms on plasma lipid variables and common demographic parameters in Greek nurses. Open Cardiovasc Med J. 2016;10:233–9.
Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240–5.
Adorni MP, Cipollari E, Favari E, Zanotti I, et al. Inhibitory effect of PCSK9 on Abca1 protein expression and cholesterol efflux in macrophages. Atherosclerosis. 2017;256:1–6.
Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008;263(3):256–73.
Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–9.
Graham MJ, Lemonidis KM, Whipple CP, et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res. 2007;48:763–7.
Chan JC, Piper DE, Cao Q, et al. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc Natl Acad Sci U S A. 2009;106:9820–5.
Clarke MC, Figg N, Maguire JJ, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–80.
Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43.
May P, Bock HH, Nofer JR. Low density receptor-related protein 1 (LRP1) promotes anti-inflammatory phenotype in murine macrophages. Cell Tissue Res. 2013;354:887–9.
Ason B, Van der Hoorn JWA, Chan J, et al. PCSK9 inhibition fails to alter hepatic LDLR, circulating cholesterol, and atherosclerosis in the absence of ApoE. J Lipid Res. 2014;55:2370–9.
Levy E, Ben Djoudi Ouadda A, Spahis S, et al. PCSK9 plays a significant role in cholesterol homeostasis and lipid transport in intestinal epithelial cells. Atherosclerosis. 2013;227:297–306.
Sharotri V, Collier DM, Olson DR, Zhou R, Snyder PM. Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9). J Biol Chem. 2012;287:19266–74.
Le MC, Kourimate S, Langhi C, et al. Proprotein convertase subtilisin kexin type 9 null mice are protected from postprandial triglyceridemia. Arterioscler Thromb Vasc Biol. 2009;29:684–90.
Poirier S, Mayer G, Benjannet S, et al. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J Biol Chem. 2008;283:2363–72.
Kawakami R, Nozato Y, Nakagami H, et al. Development of vaccine for dyslipidemia targeted to a proprotein convertase subtilisin/kexin type 9 (PCSK9) epitope in mice. PLoS One. 2018;13:e0191895.
Schuster S, Rubil S, Endres M, et al. Anti-PCSK9 antibodies inhibit pro-atherogenic mechanisms in APOE*3Leiden.CETP mice. Sci Rep. 2019;9:1–8.
Galabova G, Brunner S, Winsauer G, et al. Peptide-based anti-PCSK9 vaccines- an approach for long-term LDLc management. PLoS One. 2014;9(12):e114469.
Landlinger C, Pouwer MG, Juno C, et al. The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE*3Leiden.CETP mice. Eur Heart. 2017;38:2499–507.
Momtazi-Borojeni AA, Jaafari MR, Badiee A, Banach M, Sahebkar A. Therapeutic effect of nanoliposomal PCSK9 vaccine in a mouse model of atherosclerosis. BMC Med. 2019;17:223.
Wu D, Zhou Y, Pan Y, et al. Vaccine against PCSK9 improved renal fibrosis by regulating fatty acid β-oxidation. J Am Heart Assoc. 2020;9:e014358.
Funding
This study was funded by the Major Research Plan of the National Natural Science Foundation of China (no. 91439207) and the National Natural Science Foundation of China (nos. 81900401, 81900459, 81670461 and 81500388).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Wu, D., Pan, Y., Yang, S. et al. PCSK9Qβ-003 Vaccine Attenuates Atherosclerosis in Apolipoprotein E-Deficient Mice. Cardiovasc Drugs Ther 35, 141–151 (2021). https://doi.org/10.1007/s10557-020-07041-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-07041-6