Abstract
Purpose
Oxidative stress causes mitochondrial dysfunction in myocardial ischaemia/reperfusion (I/R) as well as in obesity. Mitochondrial depolarization triggers mitophagy to degrade damaged mitochondria, a process important for quality control. The aims of this study were to evaluate (i) the effect of I/R on mitochondrial oxidative phosphorylation and its temporal relationship with mitophagy in hearts from obese rats and their age-matched controls, and (ii) the role of oxidative stress in these processes using melatonin, a free radical scavenger.
Methods
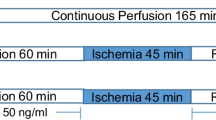
Male Wistar rats were divided into 4 groups: control (normal diet ± melatonin) and high-fat sucrose diet (HFSD ± melatonin). Rats received melatonin orally (10 mg/kg/day). After 16 weeks, hearts were removed and subjected to 40-min stabilization, and 25-min global ischaemia/10-min reperfusion for preparation of mitochondria. Mitochondrial oxidative phosphorylation was measured polarographically. Western blotting was used for evaluation of PINK1, Parkin, p62/SQSTM1 (p62) and TOM 70. Infarct size was measured using tetrazolium staining.
Results
Ischaemia and reperfusion respectively reduced and increased mitochondrial QO2 (state 3) and the ox-phos rate in both control and HFSD mitochondria, showing no major changes between the groups, while melatonin pretreatment had little effect. p62 as indicator of mitophagic flux showed up- and downregulation of mitophagy by ischaemia and reperfusion respectively, with melatonin having no significant effect. Melatonin treatment caused a significant reduction in infarct size in hearts from both control and diet groups.
Conclusions
The results suggest that I/R (i) affects mitochondria from control and HFSD hearts similarly and (ii) melatonin-induced cardioprotection is not associated with reversal of mitochondrial dysfunction or changes in the PINK1/Parkin pathway.








Similar content being viewed by others
References
Ferrari R. Importance of oxygen free radicals during ischemia and reperfusion in the experimental and clinical setting. Oxygen free radicals and the heart. Am J Cardiovasc Pathol. 1992;4:103–14.
Watts JA, Kline JA. Bench to bedside: the role of mitochondrial medicine in the pathogenesis and treatment of cellular injury. Acad Emerg Med. 2003;10:985–97.
Anzell AR, Sanderson TH. Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol Neurobiol. 2018;55:2547–64.
Saito T, Sadoshima J. Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circ Res. 2015;116:1477–90.
Hamacher-Brady A, Brady N, Loque SE, Saven MR, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–57.
Lu W, Sun J, Yoon JS, Zhang Y, Zheng L, Murphy E, et al. Mitochondrial protein PGAM5 regulates mitophagic protection against cell necroptosis. PLoS One. 2016;11:e0147792.
Kubli DA, Zhang G, Lee Y, et al. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 2013;288:915–26.
Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–87.
Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS One. 2011;6:e20975.
Ji W, Wei S, Hao P, et al. Aldehyde dehydrogenase 2 has cardioprotective effects on myocardial ischaemia/reperfusion injury via suppressing mitophagy. Front Pharmacol. 2016;7:101.
Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57:1071–7.
Putti R, Sica R, Migliaccio V, Lionetti L. Diet impact on mitochondrial bioenergetics and dynamics. Front Physiol. 2015;6:109.
Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K, et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. 2015;64:3135–45.
Zorzano A, Liesa M, Palacín M. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int J Biochem Cell Biol. 2009;41:1846–54.
Lahera V, de Las HN, López-Farré A, Manucha W, Ferder LL. Role of mitochondrial dysfunction in hypertension and obesity. Curr Hypertens Rep. 2017;19:11.
Che Y, Wang Z-P, Yuan Y, Zhang N, et al. Autophagy in a model of obesity: a ong-term high fat diet induces cardiac dysfunction. Mol Med Rep. 2015;18:3251–61.
Lochner A, Marais E, Huisamen B. Melatonin and cardioprotection against ischaemia/reperfusion injury: what’s new. A review. J Pineal Res. 2018;65:e12490.
Reiter RJ, Tan DX. Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc Res. 2003;58:10–9.
Nduhirabandi F, Du Toit EF, Blackhurst D, Marais D, Lochner A. Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against ischemic and reperfusion injury in a prediabetic model of diet-induced obesity. J Pineal Res. 2011;50:171–82.
Nduhirabandi F, Huisamen B, Strijdom H, Blackhurst D, Lochner A. Short-term melatonin consumption protects the hearts of obese rats independent of body weight and visceral adiposity. J Pineal Res. 2014;57:317–32.
Reiter RJ, Rosales-Corral S, Tan DX, et al. Melatonin as a mitochondria-targeted anti-oxidant: one of nature’s best ideas. Cell Mol Life Sci. 2017;74:3853–81.
Tan DX, Manchester LC, Qin L, Reiter RJ. Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci. 2016;17:e2124.
Petrosillo G, Colantuono G, Moro N, Ruggiero FM, Tiravanti E, di Venosa N, et al. Melatonin protects against heart ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am J Phys Heart Circ Physiol. 2009;297:H1487–93.
Lochner A, Genade S, Moolman JA. Ischemic preconditioning: infarct size is a more reliable endpoint than functional recovery. Basic Res Cardiol. 2003;98:337–46.
Sordahl LA, Besch HR Jr, Allen JC, Crow C, Lindenmayer GE, Schwartz A. Enzymatic aspects of the cardiac muscle cell: mitochondria, sarcoplasmic reticulum and noncovalent cation active transport system. Methods Achiev Exp Pathol. 1971;5:287–346.
Dube K, Dhanabalan K, Salie R, Blignaut M, Huisamen B, Lochner A. Melatonin has profound effects on mitochondrial dynamics in myocardial ischaemia/reperfusion. Heliyon. 2019;5:e02659.
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75.
Essop MF, Chan WY, Valle A, Garcia-Palmer FJ, du Toit EF. Impaired contractile function and mitochondrial respiratory capacity in response to oxygen deprivation in a rat model of prediabetes. Acta Physiol (Oxford). 2009;197:289–96.
Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Smit SE, Johnson R, Van Vuuren MA, Huisamen B. Myocardial clearance by aspalathin treatment in young, mature and obese insulin-resistant rats. Planta Med. 2018;84:75–82.
Everson F, Genis F, Ogundipe O, et al. Treatment with a fixed dose combination antiretroviral therapy drug containing tenofovir, emtricitabine and efavirenz is associated with cardioprotection in high calorie diet-induced obese rats. PLoS One. 2018;13:e0208537.
Hussein MR, Ahmed OG, Hassan AF, Ahmed MA. Intake of melatonin is associated with amelioration of physiological changes, both metabolic and morphological pathologies associated with obesity: an animal model. Int J Exp Pathol. 2007;88:19–29.
Prunet-Marcassus B, Desbazeille M, Bros A, Louche K, Delagrange P, Renard P, et al. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology. 2003;144:5347–2.
Aqil A, Rosado I, Ruiz, et al. Melatonin improves glucose homeostasis in young Zucker diabetic fatty rats. J Pineal Res. 2012;52:203–10.
Aqil A, Reiter RJ, Jimenez-Aranda A, et al. Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J Pineal Res. 2013;54:381–8.
Nduhirabandi F, Du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol Acta. 2012;205:209–23.
Bartness TJ, Wade GN. Body weight, food intake and energy regulation in exercising and melatonin-treated Siberian hamsters. Physiol Behav. 1985;35:805–8.
Wolden-Hanson T, Mitton DR, McCants RL, Yellon SM, Wilkinson CW, Matsumoto AM, et al. Daily melatonin administration to middle- aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology. 2000;141:487–97.
Angers K, Haddad N, Selmaoui B, Thibault L. Effect of melatonin on total food intake and macronutrient choice in rats. Physiol Behav. 2003;80(1):9–18.
Salie R, Huisamen B, Lochner A. High carbohydrate and high fat diets protect the heart against ischaemia/reoerfusion injury. Cardiovasc Diabetol. 2014;13:109.
Donner D, Headrick JP, Peart JN, du Toit EF. Obesity improves myocardial ischemic tolerance and RISK signalling in insulin-insensitive rats. Dis Mod Mech. 2013;6:457–66.
Pandi-Perumal SR, BaHammam AS, Ojike NI, Akinseye OA, Kendzerska T, Buttoo K, et al. Melatonin and human cardiovascular disease. J Cardiovasc Pharmacol Ther. 2017;22:122–32.
Edoute Y, van der Merwe E, Sanan D, Kotzé JC, Steinmann C, Lochner A. Normothermic ischemic arrest of the isolated working rat heart. Effects of time and reperfusion on myocardial ultrastructural, mitochondrial oxidative function and mechanical recovery. Circ Res. 1983;53:663–78.
Duncan JG. Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta. 1813;2011:1351–9.
Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME. Abel ED (2005) reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–95.
Cole MA, Murray AJ, Cochlin LE, Heather LC, McAleese S, Knight NS, et al. A high fat diet increases mitochondrial fatty acid oxidation and uncoupling to decrease efficiency in rat heart. Basic Res Cardiol. 2011;106:447–57.
Dabkowski ER, Baseler WA, Williamson CL, Powell M, Razunguzwa TT, Frisbee JC, et al. Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct proteomes. Am J Physiol Heart Circ Physiol. 2010;299:H529–40.
Koncsos G, Varga ZV, Baranyi T, et al. Diastolic dysfunction in prediabetic male rats: role of mitochondrial oxidative stress. Am J Physiol Heart Circ Physiol. 2016;311:H927–43.
Abdurrahchim D, Ciapaite J, Wessels B, et al. Cardiac diastolic dysfunction in high fat-fed mice is associated with lipotoxcicity without impairment of cardiac energetics in vivo. Biochim Biophys Acta. 1842;2014:1525–37.
Lesnefsky EJ, Chen Q, Moqhaddas S. Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–7.
Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2008;77:334–43.
Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia/reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–89.
Chen Q, Younus M, Thompson J, Hu Y, Hollander JM, Lesnefsky EJ. Intermediate metabolism and fatty acid oxidation: novel targets of electron transport chain-driven injury during ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2018;314:H787–95.
Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther. 2006;319:1405–12.
Chen Q, Vasques EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–31.
Petrosillo G, Moro N, Ruggiero FM, Paradies G. Melatonin inhibits cardiolipin peroxidation in mitochondria and prevents the mitochondrial permeability transition and cytochrome release. Free Rad Biol Med. 2009;47:969–74.
Lamont K, Nduhirabandi F, Adam T, Thomas DP, Opie LH, Lecour S. Role of melatonin, melatonin receptors, and STAT3 in the cardioprotective effect of chronic and moderate consumption of red wine. Biochem Biophys Res Comm. 2015;465:719–24.
Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang S, et al. JAK2/STAT3 activation by melatonin attenuates mitochondrial oxidative damage induced by ischemia/reperfusion injury. J Pineal Res. 2013;55:275–86.
Yu L, Gong B, Duan W, Fan C, Zhang J, Li Z, et al. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1a-SIRT3 signaling. Sci Rep. 2017;7:41337.
Gottlieb RA, Andres AM, Sin J, Taylor DPJ. Untangling autophagy measurements all fluxed up. Circ Res. 2015;116:504–14.
Xie H, Xu Q, Jia J, et al. Hydrogen sulphide protects against myocardial ischemia and reperfusion injury by activating AMP-activated protein kinase to restore autophagic flux. Biochem Biophys Res Commun. 2015;458:632–8.
Siddall HK, Yellon DM, Ong S-B, Mukherjee UA, Burke N, Hall AR, et al. Loss of PINK1 increases the heart’s vulnerability to ischemia-reperfusion injury. PLoS One. 2013;8:e62400.
Kato H, Lu Q, Rapaport D, Kozjak-Pavlovic V. Tom 70 is essential for Pink 1 import into mitochondria. PLoS One. 2013;8:e58435.
Pei H-F, Hou J-N, Wei F-P, Xue Q, Zhang F, Peng CF, et al. Melatonin attenuates postmyocardial infarction injury via increasing Tom70 expression. J Pineal Res. 2017;62:e12371.
Cruz-Topete D, List EO, Okada S, Kelder B, Kopchick JJ. Proteomic changes in the heart from diet-induced pre-diabetic mice. J Proteome. 2011;74:716–27.
Saito T, Nah J, Oka S, et al. An alternative mitophagy mediated by Rab9 protects the heart against ischemia. J Clin Invest. 2019;129:801–19.
Funding
The project was funded by the South African National Research Foundation, Grant no 93579.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
Male Wistar rats were used as experimental animals. The breeding, maintenance and feeding of these rats as well as food and water consumption, and weight monitoring were all carried out in the Central Research Facility, Faculty of Health Sciences, Stellenbosch University The study conformed to the revised South African National Standard for the Care and Use of Animals for Scientific Purposes (South African Bureau of Standards, SANS 10386, 2008) and was approved by the Committee for the ethical use of animals in research of the University of Stellenbosch (project number SU-ACUM14-00039).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhanabalan, K., Mzezewa, S., Huisamen, B. et al. Mitochondrial Oxidative Phosphorylation Function and Mitophagy in Ischaemic/Reperfused Hearts from Control and High-Fat Diet Rats: Effects of Long-Term Melatonin Treatment. Cardiovasc Drugs Ther 34, 799–811 (2020). https://doi.org/10.1007/s10557-020-06997-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-06997-9