Abstract
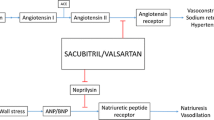
LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor (ARNI), is comprised of the angiotensin receptor blocker valsartan and the neprilysin inhibitor pro-drug sacubitril (AHU377). After oral administration, AHU377 is rapidly metabolized to the active neprilysin inhibitor LBQ657. LCZ696 exerts its effects of diuresis, natriuresis, vasodilation and aldosterone secretion inhibition through simultaneous renin-angiotensin-aldosterone system (RAAS) blockade and natriuretic peptides system (NPS) enhancement. Powerful evidence including PARAMETER and PRARDIGM-HF trials have shown that LCZ696 outperforms RAAS inhibition in treating patients with hypertension and heart failure with reduced ejection fraction (HFrEF), and is well tolerated. In addition, accumulating evidence also suggests its potential use in heart failure with preserved ejection fraction (HFpEF), chronic kidney disease (CKD), post-myocardium infarction (post-MI) and stroke. Both the FDA and CHMP have approved LCZ696 for treatment of HFrEF. Despite all this, some special issues (e.g. use in specific subgroups, adverse events, contraindications and cost-effectiveness analysis) should be considered before its implementation in clinical practice.

Similar content being viewed by others
References
Vardeny O, Tacheny T, Solomon SD. First-in-class angiotensin receptor neprilysin inhibitor in heart failure. Clin Pharmacol Ther. 2013;94(4):445–8.
Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010;50(4):401–14.
Gardner DG, Chen S, Glenn DJ, Grigsby CL. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension. 2007;49(3):419–26.
Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27(1):47–72.
Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50(25):2357–68.
Mangiafico S, Costello-Boerrigter LC, Andersen IA, Cataliotti A, Burnett Jr JC. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2013;34(12):886–93c.
Bayes-Genis A, Barallat J, Galan A, de Antonio M, Domingo M, Zamora E, et al. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J Am Coll Cardiol. 2015;65(7):657–65.
Rademaker MT, Charles CJ, Espiner EA, Nicholls MG, Richards AM, Kosoglou T. Neutral endopeptidase inhibition: augmented atrial and brain natriuretic peptide, haemodynamic and natriuretic responses in ovine heart failure. Clin Sci (Lond). 1996;91(3):283–91.
Cruden NL, Fox KA, Ludlam CA, Johnston NR, Newby DE. Neutral endopeptidase inhibition augments vascular actions of bradykinin in patients treated with angiotensin-converting enzyme inhibition. Hypertension. 2004;44(6):913–8.
Wilkinson IB, McEniery CM, Bongaerts KH, MacCallum H, Webb DJ, Cockcroft JR. Adrenomedullin (ADM) in the human forearm vascular bed: effect of neutral endopeptidase inhibition and comparison with proadrenomedullin NH2-terminal 20 peptide (PAMP). Br J Clin Pharmacol. 2001;52(2):159–64.
Erdos EG, Skidgel RA. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989;3(2):145–51.
Abassi Z, Golomb E, Keiser HR. Neutral endopeptidase inhibition increases the urinary excretion and plasma levels of endothelin. Metabolism. 1992;41(7):683–5.
McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8(1):79–84.
Bevan EG, Connell JM, Doyle J, Carmichael HA, Davies DL, Lorimer AR, et al. Candoxatril, a neutral endopeptidase inhibitor: efficacy and tolerability in essential hypertension. J Hypertens. 1992;10(7):607–13.
Ando S, Rahman MA, Butler GC, Senn BL, Floras JS. Comparison of candoxatril and atrial natriuretic factor in healthy men. Effects on hemodynamics, sympathetic activity, heart rate variability, and endothelin. Hypertension. 1995;26(6 Pt 2):1160–6.
Ferro CJ, Spratt JC, Haynes WG, Webb DJ. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation. 1998;97(23):2323–30.
Trippodo NC, Fox M, Monticello TM, Panchal BC, Asaad MM. Vasopeptidase inhibition with omapatrilat improves cardiac geometry and survival in cardiomyopathic hamsters more than does ACE inhibition with captopril. J Cardiovasc Pharmacol. 1999;34(6):782–90.
Rouleau JL, Pfeffer MA, Stewart DJ, Isaac D, Sestier F, Kerut EK, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356(9230):615–20.
Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the omapatrilat cardiovascular treatment vs. enalapril (OCTAVE) trial. Am J Hypertens. 2004;17(2):103–11.
Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the omapatrilat versus enalapril randomized trial of utility in reducing events (OVERTURE)[J]. Circulation. 2002;106(8):920–6.
Messerli FH, Nussberger J. Vasopeptidase inhibition and angio-oedema. Lancet. 2000;356(9230):608–9.
Fryer RM, Segreti J, Banfor PN, Widomski DL, Backes BJ, Lin CW, et al. Effect of bradykinin metabolism inhibitors on evoked hypotension in rats: rank efficacy of enzymes associated with bradykinin-mediated angioedema. Br J Pharmacol. 2008;153(5):947–55.
Hegde LG, Yu C, Renner T, Thibodeaux H, Armstrong SR, Park T, et al. Concomitant angiotensin AT1 receptor antagonism and neprilysin inhibition produces omapatrilat-like antihypertensive effects without promoting tracheal plasma extravasation in the rat. J Cardiovasc Pharmacol. 2011;57(4):495–504.
Feng L, Karpinski PH, Sutton P, Liu Y, Hook DF, Hu B, et al. LCZ696: a dual-acting sodium supramolecular complex. Tetrahedron Lett. 2012;53(3):275–6.
Baxter GF. The natriuretic peptides. Basic Res Cardiol. 2004;99(2):71–5.
Gan L, Langenickel T, Petruck J, Kode K, Rajman I, Chandra P, et al. Effects of age and sex on the pharmacokinetics of LCZ696, an angiotensin receptor neprilysin inhibitor. J Clin Pharmacol. 2016;56(1):78–86.
Han Y, Ayalasomayajula S, Pan W, Yang F, Yuan Y, Langenickel T, et al. Pharmacokinetics, safety and tolerability of sacubitril/valsartan (LCZ696) after single-dose Administration in Healthy Chinese Subjects. Eur J Drug Metab Pharmacokinet. 2016;1-8
Akahori M, Ayalasomayajula S. Pharmacokinetics after single ascending dose, food effect, and safety of sacubitril/valsartan (LCZ696), an angiotensin receptor and neprilysin inhibitor, in healthy Japanese subjects. Eur J Drug Metab Pharmacokinet. 2016;1–10.
Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep. 2014;11(4):404–15.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–60.
Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More 'malignant' than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3(3):315–22.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey Jr DE, Colvin MM, et al. ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68(13):1476–88.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54–61.
Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36(30):1990–7.
Claggett B, Packer M, McMurray JJ, Swedberg K, Rouleau J, Zile MR, et al. Estimating the long-term treatment benefits of sacubitril-valsartan. N Engl J Med. 2015;373(23):2289–90.
Desai AS, Claggett BL, Packer M, Zile MR, Rouleau JL, Swedberg K, et al. Influence of sacubitril/valsartan (LCZ696) on 30-day readmission after heart failure hospitalization. J Am Coll Cardiol. 2016;68(3):241–8.
Solomon SD, Claggett B, Desai AS, Packer M, Zile M, Swedberg K, et al. Influence of ejection fraction on outcomes and efficacy of sacubitril/valsartan (LCZ696) in heart failure with reduced ejection fraction: the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure (PARADIGM-HF) trial. Circ Heart Fail. 2016;9(3):e002744.
Vardeny O, Claggett B, McMurray JJV, Packer M, Rouleau J, Teerlink JR, et al. Efficacy of LCZ696 persists at lower than target doses in the PARADIGM-HF trial. J Card Fail. 2016;21(8):S9–S10.
Jhund PS, Fu M, Bayram E, Chen CH, Negrusz-Kawecka M, Rosenthal A, et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015;36(38):2576–84.
Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, et al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail. 2016;9(1):e002560.
Simpson J, Jhund PS, Cardoso JS, Martinez F, Mosterd A, Ramires F, et al. Comparing LCZ696 with enalapril according to baseline risk using the MAGGIC and EMPHASIS-HF risk scores. J Am Coll Cardiol. 2015;66(19):2059–71.
McMurray J, Packer M, Desai A, Gong J, Greenlaw N, Lefkowitz M, et al. A putative placebo analysis of the effects of LCZ696 on clinical outcomes in heart failure. Eur Heart J. 2015;36(7):434–9.
Smith GL, Masoudi FA, Vaccarino V, Radford MJ, Krumholz HM. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol. 2003;41(9):1510–8.
Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387–95.
Jhund PS, Claggett B, Packer M, Zile MR, Voors AA, Pieske B, et al. Independence of the blood pressure lowering effect and efficacy of the angiotensin receptor neprilysin inhibitor, LCZ696, in patients with heart failure with preserved ejection fraction: an analysis of the PARAMOUNT trial. Eur J Heart Fail. 2014;16(6):671–7.
Jhund PS, Claggett BL, Voors AA, Zile MR, Packer M, Pieske BM, et al. Elevation in high-sensitivity troponin T in heart failure and preserved ejection fraction and influence of treatment with the angiotensin receptor neprilysin inhibitor LCZ696. Circ Heart Fail. 2014;7(6):953–9.
Novartis Pharmaceuticals. Efficacy and safety of LCZ696 compared to valsartan, on morbidity and mortality in heart failure patients with preserved ejection fraction (PARAGON-HF). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2013 Aug 8- [cited 2016 Jul 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT01920711.
Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375(9722):1255–66.
Kario K, Sun N, Chiang FT, Supasyndh O, Baek SH, Inubushi-Molessa A, et al. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension. 2014;63(4):698–705.
Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65(2):252–6.
Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J, Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61(1):112–9.
Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension. 2011;58(5):839–46.
Hashimoto J, Ito S. Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: potential implication for retrograde embolic stroke in hypertension. Hypertension. 2013;62(3):542–9.
Karras A, Haymann JP, Bozec E, Metzger M, Jacquot C, Maruani G, et al. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60(6):1451–7.
Pu Q, Brassard P, Javeshghani DM, Iglarz M, Webb RL, Amiri F, et al. Effects of combined AT1 receptor antagonist/NEP inhibitor on vascular remodeling and cardiac fibrosis in SHRSP. J Hypertens. 2008;26(2):322–33.
Kusaka H, Sueta D, Koibuchi N, Hasegawa Y, Nakagawa T, Lin B, et al. LCZ696, angiotensin II receptor-neprilysin inhibitor, ameliorates high-salt-induced hypertension and cardiovascular injury more than valsartan alone. Am J Hypertens. 2015;28(12):1409–17.
Williams B, Cockcroft JR, Kario K, et al. Principal results of the prospective comparison of angiotensin receptor neprilysin inhibitor with angiotensin receptor blocker measuring arterial stiffness in the elderly (PARAMETER) study. European Society of Cardiology 2015 Congress:London, UK. Abstract 4143. August 31, 2015
Kario K, Tamaki Y, Okino N, Gotou H, Zhu M, Zhang J. LCZ696, a first-in-class angiotensin receptor-neprilysin inhibitor: the first clinical experience in patients with severe hypertension. J Clin Hypertens (Greenwich). 2016;18(4):308–14.
Lazzeri C, Franchi F, Porciani C, Fronzaroli C, Casini Raggi V, De Feo ML, et al. Systemic hemodynamics and renal function during brain natriuretic peptide infusion in patients with essential hypertension. Am J Hypertens. 1995;8(8):799–807.
Franco-Saenz R, Somani P, Mulrow PJ. Effect of atrial natriuretic peptide (8-33-Met ANP) in patients with hypertension. Am J Hypertens. 1992;5(5 Pt 1):266–75.
Predel HG, Schulte-Vels O, Glanzer K, Meyer-Lehnert H, Kramer HJ. Atrial natriuretic peptide in patients with essential hypertension. Hemodynamic, renal, and hormonal responses. Am J Hypertens. 1991;4(11):871–9.
Singer DR, Markandu ND, Buckley MG, Miller MA, Sugden AL, Sagnella GA, et al. Prolonged decrease in blood pressure after atrial natriuretic peptide infusion in essential hypertension: a new anti-pressor mechanism? Clin Sci (Lond). 1989;77(3):253–8.
Cataliotti A, Costello-Boerrigter LC, Chen HH, Textor SC, Burnett Jr JC. Sustained blood pressure-lowering actions of subcutaneous B-type natriuretic peptide (nesiritide) in a patient with uncontrolled hypertension. Mayo Clin Proc. 2012;87(4):413–5.
Patel P, Chen HH. Natriuretic peptides as a novel target in resistant hypertension. Curr Hypertens Rep. 2015;17(3):18.
Benigni A, Zoja C, Zatelli C, Corna D, Longaretti L, Rottoli D, et al. Vasopeptidase inhibitor restores the balance of vasoactive hormones in progressive nephropathy. Kidney Int. 2004;66(5):1959–65.
Cao Z, Burrell LM, Tikkanen I, Bonnet F, Cooper ME, Gilbert RE. Vasopeptidase inhibition attenuates the progression of renal injury in subtotal nephrectomized rats. Kidney Int. 2001;60(2):715–21.
Seymour AA, Asaad MM, Lanoce VM, Langenbacher KM, Fennell SA, Rogers WL. Systemic hemodynamics, renal function and hormonal levels during inhibition of neutral endopeptidase 3.4.24.11 and angiotensin-converting enzyme in conscious dogs with pacing-induced heart failure. J Pharmacol Exp Ther. 1993;266(2):872–83.
Cataliotti A, Boerrigter G, Chen HH, Jougasaki M, Costello LC, Tsuruda T, et al. Differential actions of vasopeptidase inhibition versus angiotensin-converting enzyme inhibition on diuretic therapy in experimental congestive heart failure. Circulation. 2002;105(5):639–44.
Voors AA, Gori M, Liu LC, Claggett B, Zile MR, Pieske B, et al. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2015;17(5):510–7.
Wang BH, von Lueder TG, Kompa AR, Huang L, Webb R, Jordaan P, et al. Combined angiotensin receptor blockade and neprilysin inhibition attenuates angiotensin-II mediated renal cellular collagen synthesis. Int J Cardiol. 2015;186:104–5.
Bodey F, Hopper I, Krum H. Neprilysin inhibitors preserve renal function in heart failure. Int J Cardiol. 2015;179:329–30.
ISRCTN Registry. London: current control trials, c/o BioMed Centrol. 2010. UK Heart and Renal Protection (UK HARP-III); 2014 Jan 20[cited 2016 Jul 21]; Available from: http://www.isrctn.com/ISRCTN11958993.
von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. 2015;8(1):71–8.
Marshall JM, Chamberlin KW. New drug review: FDA approves sacubitril/valsartan to treat heart failure. Drug Topics. 2015;159(11)
European Medicines Agency. New medicine to treat heart failure recommended for approval. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2015/09/WC500194185.pdf. Accessed Sep 25, 2015.
Moe GW, Ezekowitz JA, O'Meara E, Lepage S, Howlett JG, Fremes S, et al. The 2014 Canadian cardiovascular society heart failure management guidelines focus update: anemia, biomarkers, and recent therapeutic trial implications. Can J Cardiol. 2015;31(1):3–16.
Bayes-Genis A. Neprilysin in heart failure: from oblivion to center stage. JACC Heart Failure. 2015;3(8):637–40.
Vodovar N, Seronde MF, Laribi S, Gayat E, Lassus J, Januzzi Jr JL, et al. Elevated plasma B-type natriuretic peptide concentrations directly inhibit circulating neprilysin activity in heart failure. JACC Heart Failure. 2015;3(8):629–36.
Dec GW. LCZ696 (sacubitril/valsartan): can We predict who will benefit? J Am Coll Cardiol. 2015;66(19):2072–4.
Prenner SB, Shah SJ, Yancy CW. Role of angiotensin receptor-neprilysin inhibition in heart failure. Curr Atheroscler Rep. 2016;18(8):1–10.
Senni M, McMurray JJ, Wachter R, McIntyre HF, Reyes A, Majercak I, et al. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double-blind, randomized comparison of two uptitration regimens. Eur J Heart Fail. (2016)
Filippatos G, Farmakis D, Parissis J, Lekakis J. Drug therapy for patients with systolic heart failure after the PARADIGM-HF trial: in need of a new paradigm of LCZ696 implementation in clinical practice. BMC Med. 2015;1(13):1–3.
Desai AS, Solomon S, Claggett B, McMurray JJ, Rouleau J, Swedberg K, et al. Factors associated with Noncompletion during the run-in period before randomization and influence on the estimated benefit of LCZ696 in the PARADIGM-HF trial. Circ Heart Fail. 2016;9(6):e002735.
Ayalasomayajula S. Jordaan, Pierre, Parasar, Albrecht, et al. abstract 448: assessment of drug interaction potential between LCZ696 and warfarin. Hypertension. 2013;62(Suppl 1):A448–8.
Ayalasomayajula S, Jordaan P, Pal P, Albrecht D, Langenickel T, Sunkara G, et al. Abstract 449: assessment of pharmacokinetic drug interaction between LCZ696 and digoxin. Hypertension. 2013;62(Suppl 1):A449–9.
Ayalasomayajula S, Pan W, Han Y, Yang F, Langenickel T, Pal P, et al. Assessment of drug-drug interaction potential between atorvastatin and LCZ696, a novel angiotensin receptor neprilysin inhibitor, in healthy Chinese male subjects. Eur J Drug Metab Pharmacokinet. 2016;1-10
Hsiao HL, Langenickel TH, Greeley M, Roberts J, Zhou W, Pal P, et al. Pharmacokinetic drug-drug interaction assessment between LCZ696, an angiotensin receptor neprilysin inhibitor, and hydrochlorothiazide, amlodipine, or carvedilol. Clin Pharmacol Drug Dev. 2015;4(6):407–17.
Gan L, Jiang X, Mendonza A, Swan T, Reynolds C, Nguyen J, et al. Pharmacokinetic drug-drug interaction assessment of LCZ696 (an angiotensin receptor neprilysin inhibitor) with omeprazole, metformin or levonorgestrel-ethinyl estradiol in healthy subjects. Clin Pharmacol Drug Dev. 2016;5(1):27–39.
King JB, Bress AP, Reese AD, Munger MA. Neprilysin inhibition in heart failure with reduced ejection fraction: a clinical review. Pharmacotherapy. 2015;35(9):823–37.
Fonarow GC, Hernandez AF, Solomon SD, Yancy CW. Potential mortality reduction with optimal implementation of angiotensin receptor neprilysin inhibitor therapy in heart failure. JAMA Cardiol. 2016;1(6):1–4.
Gaziano TA, Fonarow GC, Claggett B, Chan WW, Deschaseaux-Voinet C, Turner SJ, et al. Cost-effectiveness analysis of Sacubitril/Valsartan vs Enalapril in patients with heart failure and reduced ejection fraction. JAMA Cardiol. 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
We confirm that there is no funding support in this manuscript and that we did not receive any support from the sponsor Novartis. We declare that we are the sole authors of the manuscript and the article was not written by any agency or bureau with any external financial support.
Conflict of Interest
The authors declare that they do not have any conflict of interest with respect to this manuscript.
Ethical Approval
This article does not contain studies with human participants or animals performed by any of the authors.
Additional information
L. M. Lin and M. F. Wu contributed equally to this article.
Rights and permissions
About this article
Cite this article
Lin, L.M., Wu, Y., Wu, M.F. et al. Focus on the Novel Cardiovascular Drug LZC696: from Evidence to Clinical Consideration. Cardiovasc Drugs Ther 30, 623–633 (2016). https://doi.org/10.1007/s10557-016-6699-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-016-6699-5