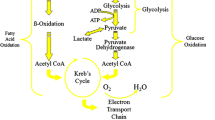
Abstract
Approaches to the pharmacotherapy of angina pectoris have previously centred on the concept that a transient imbalance between myocardial oxygen “demand” and supply within the myocardium can best be addressed by reducing demand (for example, with β–adrenoceptor antagonist) or by increasing availability of blood (via coronary vasomotor reactivity adjustment or coronary revascularization). However, this principle is potentially challenged by the emergence of cases of angina unsuitable for such therapies (for example because of concomitant severe systolic heart failure) and by the recognition that impaired myocardial energetics may precipitate angina in the absence of fixed or variable coronary obstruction (for example in hypertrophic cardiomyopathy). The past 20 years have seen the re-emergence of a class of anti-anginal agents which act primarily by improving efficiency of myocardial oxygen utilization, and thus can correct impaired energetics, simultaneously treating angina and heart failure symptoms. We review the principles underlying the safe use of such agents, beginning with the prototype drug perhexiline maleate, which despite complex pharmacokinetics and potential hepato- or neuro-toxicity has emerged as an attractive management option in many “complicated” cases of angina pectoris.


Similar content being viewed by others
References
Liedtke AJ, Renstrom B, Nellis SH, Hall JL, Stanley WC. Mechanical and metabolic functions in pig hearts after 4 days of chronic coronary stenosis. J Am Coll Cardiol. 1995;26:815–25.
Liedtke AJ, Renstrom B, Nellis SH, Subramanian R, Woldegiorgis G. Myocardial metabolism in chronic reperfusion after nontransmural infarction in pig hearts. Am J Phys. 1993;265:H1614–22.
Turer AT, Stevens RD, Bain JR, Muehlbauer MJ, van der Westhuizen J, Mathew JP, Schwinn DA, Glower DD, Newgard CB, Podgoreanu MV. Metabolomic profiling reveals distinct patterns of myocardial substrate use in humans with coronary artery disease or left ventricular dysfunction during surgical ischemia/reperfusion. Circulation. 2009;119:1736–46.
Shannon RP, Komamura K, Shen YT, Bishop SP, Vatner SF. Impaired regional subendocardial coronary flow reserve in conscious dogs with pacing-induced heart failure. Am J Phys. 1993;265:H801–9.
Shivu GN, Abozguia K, Phan TT, Ahmed I, Henning A, Frenneaux M. (31)P magnetic resonance spectroscopy to measure in vivo cardiac energetics in normal myocardium and hypertrophic cardiomyopathy: Experiences at 3 T. Eur J Radiol. 2010;73:255–9.
Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, von Kienlin M, Harre K, Hahn D, Neubauer S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol. 2002;40:1267–74.
Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, Mader H, Kromer EP, Riegger GA, Lackner K, et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86:1810–8.
Fillmore N, Mori J, Lopaschuk GD. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol. 2014;171:2080–90.
Neubauer S. The failing heart–an engine out of fuel. N Engl J Med. 2007;356:1140–51.
McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14.
McGarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977;60:265–70.
McGarry JD, Mannaerts GP, Foster DW. Characteristics of fatty acid oxidation in rat liver homogenates and the inhibitory effect of malonyl-CoA. Biochim Biophys Acta. 1978;530:305–13.
Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins–novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–53.
Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–26.
Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53.
Jeoung NH. Pyruvate dehydrogenase Kinases: therapeutic targets for diabetes and Cancers. Diabetes Metab J. 2015;39:188–97.
Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–9.
Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–85.
Kornfeld OS, Hwang S, Disatnik MH, Chen CH, Qvit N, Mochly-Rosen D. Mitochondrial reactive oxygen species at the heart of the Matter: new therapeutic Approaches for cardiovascular diseases. Circ Res. 2015;116:1783–99.
Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13.
Burkart V, Wang ZQ, Radons J, Heller B, Herceg Z, Stingl L, Wagner EF, Kolb H. Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozocin. Nat Med. 1999;5:314–9.
Howell NJ, Ashrafian H, Drury NE, Ranasinghe AM, Contractor H, Isackson H, Calvert M, Williams LK, Freemantle N, Quinn DW, Green D, Frenneaux M, Bonser RS, Mascaro JG, Graham TR, Rooney SJ, Wilson IC, Pagano D. Glucose-insulin-potassium reduces the incidence of low cardiac output episodes after aortic valve replacement for aortic stenosis in patients with left ventricular hypertrophy: results from the Hypertrophy, Insulin, Glucose, and Electrolytes (HINGE) trial. Circulation. 2011;123:170–7.
Bachmann E, Weber E. Biochemical mechanisms of oxfenicine cardiotoxicity. Pharmacology. 1988;36:238–48.
Cabrero A, Merlos M, Laguna JC, Carrera MV. Down-regulation of acyl-CoA oxidase gene expression and increased NF-kappaB activity in etomoxir-induced cardiac hypertrophy. J Lipid Res. 2003;44:388–98.
Gunther J, Wagner K, Theres H, Schimke I, Born A, Scholz H, Vetter R. Myocardial contractility after infarction and carnitine palmitoyltransferase I inhibition in rats. Eur J Pharmacol. 2000;406:123–6.
Turcani M, Rupp H. Etomoxir improves left ventricular performance of pressure-overloaded rat heart. Circulation. 1997;96:3681–6.
Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86:580–8.
Clarke B, Wyatt KM, McCormack JG. Ranolazine increases active pyruvate dehydrogenase in perfused normoxic rat hearts: evidence for an indirect mechanism. J Mol Cell Cardiol. 1996;28:341–50.
Kennedy JA, Horowitz JD. Effect of trimetazidine on carnitine palmitoyltransferase-1 in the rat heart. Cardiovasc Drugs Ther. 1998;12:359–63.
Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, Alehagen U, Steurer G, Littarru GP. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2:641–9.
Hudak WJ, Lewis RE, Kuhn WL. Cardiovascular pharmacology of perhexiline. J Pharmacol Exp Ther. 1970;173:371–82.
Cho YW, Belej M, Aviado DM. Pharmacology of a new antianginal drug: perhexiline. I. Coronary circulation and myocardial metabolism. Chest. 1970;58:577–81.
Roberts LN, Mason GP. Clinical trial of a new antianginal drug: perhexiline maleate. J Clin Pharmacol New Drugs. 1972;12:342–8.
Burns-Cox CJ, Chandrasekhar KP, Ikram H, Peirce TH, Pilcher J, Quinlan CD, Rees JR. Clinical evaluation of perhexiline maleate in patients with angina pectoris. Br Med J. 1971;4:586–8.
Cole PL, Beamer AD, McGowan N, Cantillon CO, Benfell K, Kelly RA, Hartley LH, Smith TW, Antman EM. Efficacy and safety of perhexiline maleate in refractory angina. A double-blind placebo-controlled clinical trial of a novel antianginal agent. Circulation. 1990;81:1260–70.
Ling LH, Chik W, Averbuj P, Pati PK, Sverdlov AL, Ngo DT, Morris RG, Sallustio BC, Horowitz JD. Effects of aging, renal dysfunction, left ventricular systolic impairment, and weight on steady state pharmacokinetics of perhexiline. Ther Drug Monit. 2011;33:251–6.
Bourrat C, Viala JJ, Guastala JP. Letter: Peripheral neuropathy after prolonged adsorption of perhexiline maleate. 2 cases. Nouv Press Med. 1975;4:2528.
Fraser DM, Campbell IW, Miller HC. Peripheral and autonomic neuropathy after treatment with perhexiline maleate. Br Med J. 1977;2:675–6.
Le Menn G, Mabin D, Penther P. Slow and incomplete regression of peripheral neuropathy due to perhexiline maleate. Ann Cardiol Angeiol (Paris). 1977;26:149–50.
Nicolas G, Delobel R, Feve JR, Rozo L. Peripheral neuropathy after perhexilene maleate administration. Ann Med Interne (Paris). 1976;127:607–10.
Dawes P, Moulder C. Perhexiline hepatitis and HLA-B8. Lancet. 1982;2:109.
Fardeau M, Tome FM, Simon P. Muscle and nerve changes induced by perhexiline maleate in man and mice. Muscle Nerve. 1979;2:24–36.
Forbes GB, Rake MO, Taylor DJ. Liver damage due to perhexiline maleate. J Clin Pathol. 1979;32:1282–5.
Singlas E, Goujet MA, Simon P. Pharmacokinetics of perhexiline maleate in anginal patients with and without peripheral neuropathy. Eur J Clin Pharmacol. 1978;14:195–201.
Wright GJ, Leeson GA, Zeiger AV, Lang JF. Proceedings: the absorption, excretion and metabolism of perhexiline maleate by the human. Postgrad Med J. 1973;49(Suppl 3):8–15.
Lhermitte F, Fardeau M, Chedru F, Mallecourt J. Polyneuropathy after perhexiline maleate therapy. Br Med J. 1976;1:1256.
Shah RR, Oates NS, Idle JR, Smith RL, Lockhart JD. Impaired oxidation of debrisoquine in patients with perhexiline neuropathy. Br Med J (Clin Res Ed). 1982;284:295–9.
Horowitz JD, Sia ST, Macdonald PS, Goble AJ, Louis WJ. Perhexiline maleate treatment for severe angina pectoris–correlations with pharmacokinetics. Int J Cardiol. 1986;13:219–29.
Sallustio BC, Westley IS, Morris RG. Pharmacokinetics of the antianginal agent perhexiline: relationship between metabolic ratio and steady-state dose. Br J Clin Pharmacol. 2002;54:107–14.
Bergey JL, McCallum JD, Nocella K. Antiarrhythmic evaluation of verapamil, nifedipine, perhexiline and skf 525-A in four canine models of cardiac arrhythmias. Eur J Pharmacol. 1981;70:331–43.
Fleckenstein-Grun GF, Byon A, Kim YK, K.W. Mechanism of action of Ca++ antagonists in the treatment of coronary disease wiht special reference to perhexiline maleate.. Perhexiline maleate. Proceedings of a Symposium. Amsterdam. Excerpta Medica. 1978:1–22.
Barry WH, Horowitz JD, Smith TW. Comparison of negative inotropic potency, reversibility, and effects on calcium influx of six calcium channel antagonists in cultured myocardial cells. Br J Pharmacol. 1985;85:51–9.
Vaughan Williams EM. Antiarrhythmic action and puzzle of perhexiline. London: Academic Press; 1980.
Jeffrey FM, Alvarez L, Diczku V, Sherry AD, Malloy CR. Direct evidence that perhexiline modifies myocardial substrate utilization from fatty acids to lactate. J Cardiovasc Pharmacol. 1995;25:469–72.
Kennedy JA, Unger SA, Horowitz JD. Inhibition of carnitine palmitoyltransferase-1 in rat heart and liver by perhexiline and amiodarone. Biochem Pharmacol. 1996;52:273–80.
Yin X, Dwyer J, Langley SR, Mayr U, Xing Q, Drozdov I, Nabeebaccus A, Shah AM, Madhu B, Griffiths J, Edwards LM, Mayr M. Effects of perhexiline-induced fuel switch on the cardiac proteome and metabolome. J Mol Cell Cardiol. 2013;55:27–30.
S. Dally, G. Lagier, R. Assan, and M. Gaultier. Hypoglycemia in 2 patients treated with perhexiline maleate. Nouv Press Med 1977; 6: 1643–4, 1649.
Unger SA, Kennedy JA, McFadden-Lewis K, Minerds K, Murphy GA, Horowitz JD. Dissociation between metabolic and efficiency effects of perhexiline in normoxic rat myocardium. J Cardiovasc Pharmacol. 2005;46:849–55.
Kennedy JA, Beck-Oldach K, McFadden-Lewis K, Murphy GA, Wong YW, Zhang Y, Horowitz JD. Effect of the anti-anginal agent, perhexiline, on neutrophil, valvular and vascular superoxide formation. Eur J Pharmacol. 2006;531:13–9.
Willoughby SR, Chirkov YY, Kennedy JA, Murphy GA, Chirkova LP, Horowitz JD. Inhibition of long-chain fatty acid metabolism does not affect platelet aggregation responses. Eur J Pharmacol. 1998;356:207–13.
Guo Y, Fan Y, Zhang J, Lomberk GA, Zhou Z, Sun L, Mathison AJ, Garcia-Barrio MT, Zhang J, Zeng L, Li L, Pennathur S, Willer CJ, Rader DJ, Urrutia R, Chen YE. Perhexiline activates KLF14 and reduces atherosclerosis by modulating ApoA-I production. J Clin Invest. 2015;125:3819–30.
Stewart S, Voss DW, Northey DL, Horowitz JD. Relationship between plasma perhexiline concentration and symptomatic status during short-term perhexiline therapy. Ther Drug Monit. 1996;18:635–9.
Phuong H, Choi BY, Chong CR, Raman B, Horowitz JD. Can perhexiline be utilized without long-term toxicity? A clinical practice audit. Ther Drug Monit. 2015;38:73–8.
Unger SA, Robinson MA, Horowitz JD. Perhexiline improves symptomatic status in elderly patients with severe aortic stenosis. Aust NZ J Med. 1997;27:24–8.
Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Fraser AG, Clarke K, Frenneaux M. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112:3280–8.
Abozguia K, Elliott P, McKenna W, Phan TT, Nallur-Shivu G, Ahmed I, Maher AR, Kaur K, Taylor J, Henning A, Ashrafian H, Watkins H, Frenneaux M. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation. 2010;122:1562–9.
Acknowledgment
Cher-Rin Chong is a recipient of National Health and Medical Research Council of Australia Dora Lush Biomedical Research Postgraduate Scholarship (APP1075767).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chong, CR., Sallustio, B. & Horowitz, J.D. Drugs that Affect Cardiac Metabolism: Focus on Perhexiline. Cardiovasc Drugs Ther 30, 399–405 (2016). https://doi.org/10.1007/s10557-016-6664-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-016-6664-3