Abstract
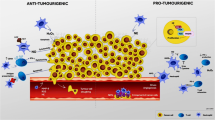
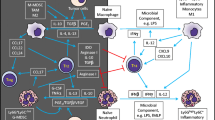
Cancer is considered a major public health concern worldwide and is characterized by an uncontrolled division of abnormal cells. The human immune system recognizes cancerous cells and induces innate immunity to destroy those cells. However, sustained tumors may protect themselves by developing immune escape mechanisms through multiple soluble and cellular mediators. Neutrophils are the most plenteous leukocytes in the human blood and are crucial for immune defense in infection and inflammation. Besides, neutrophils emancipate the antimicrobial contents, secrete different cytokines or chemokines, and interact with other immune cells to combat and successfully kill cancerous cells. Conversely, many clinical and experimental studies signpost that being a polarized and heterogeneous population with plasticity, neutrophils, particularly their subpopulations, act as a modulator of cancer development by promoting tumor metastasis, angiogenesis, and immunosuppression. Studies also suggest that tumor infiltrating macrophages, neutrophils, and other innate immune cells support tumor growth and survival. Additionally, neutrophils promote tumor cell invasion, migration and intravasation, epithelial to mesenchymal transition, survival of cancer cells in the circulation, seeding, and extravasation of tumor cells, and advanced growth and development of cancer cells to form metastases. In this manuscript, we describe and review recent studies on the mechanisms for neutrophil recruitment, activation, and their interplay with different immune cells to promote their pro-tumorigenic functions. Understanding the detailed mechanisms of neutrophil-tumor cell interactions and the concomitant roles of other immune cells will substantially improve the clinical utility of neutrophils in cancer and eventually may aid in the identification of biomarkers for cancer prognosis and the development of novel therapeutic approaches for cancer treatment.





Similar content being viewed by others
References
Hanahan, D., & Robert, A. (2011). Weinberg, Hallmarks of cancer: The next generation. Cell, 144(5), 646–674.
Kolaczkowska, E., & Kubes, P. (2013). Neutrophil recruitment and function in health and inflammation. Nature Reviews Immunology, 13(3), 159–175.
Beyrau, M., J.V. Bodkin, and S. Nourshargh, Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biology, 2012. 2(11): p. 120134.
Sagiv, J. Y., et al. (2015). Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Reports, 10(4), 562–573.
Fridlender, Z. G., et al. (2009). Polarization of tumor-associated neutrophil phenotype by TGF-β: & #x201c;N1” versus & #x201c;N2”. TAN. Cancer Cell, 16(3), 183–194.
Fridlender, Z. G., & Albelda, S. M. (2012). Tumor-associated neutrophils: Friend or foe? Carcinogenesis, 33(5), 949–955.
Wculek, S. K., & Malanchi, I. (2015). Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature, 528(7582), 413–417.
ME Shaul et al 2020 Circulating neutrophil subsets in advanced lung cancer patients exhibit unique immune signature and relate to prognosis. 34 3 4204 4218
Schernberg, A., et al., Neutrophilia as prognostic biomarker in locally advanced stage III lung cancer. PLOS ONE, 2018. 13(10): p. e0204490.
Soto-Perez-de-Celis, E., et al. (2017). Tumor-associated neutrophils in breast cancer subtypes. Asian Pacific journal of cancer prevention: APJCP, 18(10), 2689–2693.
Governa, V., et al. (2017). The interplay between neutrophils and CD8<sup>+</sup> T cells improves survival in human colorectal cancer. Clinical Cancer Research, 23(14), 3847.
S Costa et al 2019 Recent advances on the crosstalk between neutrophils and B or T lymphocytes. 156 1 23 32
Rakic, A., Beaudry, P., & Mahoney, D. J. (2018). The complex interplay between neutrophils and cancer. Cell and Tissue Research, 371(3), 517–529.
Joyce, R. A., Hartmann, O., & Chervenick, P. A. (1979). Splenic granulopoiesis in mice following administration of cyclophosphamide. Cancer Research, 39(1), 215–218.
Rosales, C., Neutrophil: a cell with many roles in inflammation or several cell types? 2018. 9(113).
Pillay, J., et al., In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood, 2010. 116(4): p. 625–7.
Ocana, A., et al. (2017). Neutrophils in cancer: Prognostic role and therapeutic strategies. Molecular Cancer, 16(1), 137.
Coffelt, S. B., Wellenstein, M. D., & de Visser, K. E. (2016). Neutrophils in cancer: Neutral no more. Nature Reviews Cancer, 16(7), 431–446.
Olsson, A. K., & Cedervall, J. (2016). NETosis in cancer - platelet-neutrophil crosstalk promotes tumor-associated pathology. Frontiers in Immunology, 7, 373.
Stojkov, D., et al. (2017). ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. Journal of Cell Biology, 216(12), 4073–4090.
Gullberg, U., et al., Biosynthesis, processing and sorting of neutrophil proteins: insight into neutrophil granule development. 1997. 58(3): p. 137–153.
Wu, M., et al. (2020). Neutrophil: A new player in metastatic cancers. Frontiers in immunology, 11, 565165–565165.
Borregaard, N., Sørensen, O. E., & Theilgaard-Mönch, K. (2007). Neutrophil granules: A library of innate immunity proteins. Trends in Immunology, 28(8), 340–345.
Sheshachalam, A., et al., Granule protein processing and regulated secretion in neutrophils. 2014. 5(448).
Reggiani, F., et al., Adipose progenitor cell secretion of GM-CSF and MMP9 promotes a stromal and immunological microenvironment that supports breast cancer progression. 2017. 77(18): p. 5169–5182.
Aalinkeel, R., et al. (2011). Overexpression of MMP-9 contributes to invasiveness of prostate cancer cell line LNCaP. Immunological Investigations, 40(5), 447–464.
Joseph, C., et al. (2020). Elevated MMP9 expression in breast cancer is a predictor of shorter patient survival. Breast cancer research and treatment, 182(2), 267–282.
P Scapini et al 2016 Human neutrophils in the saga of cellular heterogeneity: Insights and open questions. 273 1 48 60
Mishalian, I., Granot, Z., & Fridlender, Z. G. (2017). The diversity of circulating neutrophils in cancer. Immunobiology, 222(1), 82–88.
Masucci, M.T., M. Minopoli, and M.V. Carriero, Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. 2019. 9(1146).
Shojaei, F., et al. (2007). Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature, 450(7171), 825–831.
Kessenbrock, K., Plaks, V., & Werb, Z. (2010). Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell, 141(1), 52–67.
Scapini, P., & Cassatella, M. A. (2014). Social networking of human neutrophils within the immune system. Blood, 124(5), 710–719.
L Andzinski et al 2016 Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. 138 8 1982 1993
Zou, J.-M., et al., IL-35 induces N2 phenotype of neutrophils to promote tumor growth. 2017. 8(20).
Qin, F., et al. (2020). Anti-TGF-β attenuates tumor growth via polarization of tumor associated neutrophils towards an anti-tumor phenotype in colorectal cancer. Journal of Cancer, 11(9), 2580–2592.
Caruso, R. A., et al. (2002). Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in Northern Italy. Modern Pathology, 15(8), 831–837.
Sconocchia, G., et al., Tumor infiltration by FcγRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. 2011. 128(11): p. 2663–2672.
Millrud, C.R., et al., NET-producing CD16high CD62Ldim neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. 2017. 140(11): p. 2557–2567.
Singhal, S., et al. (2016). Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell, 30(1), 120–135.
Humbert, M., et al., Intratumoral CpG-B promotes antitumoral neutrophil, cDC, and T-cell cooperation without reprograming tolerogenic pDC. 2018. 78(12): p. 3280–3292.
Stoppacciaro, A., et al. (1993). Regression of an established tumor genetically modified to release granulocyte colony-stimulating factor requires granulocyte-T cell cooperation and T cell-produced interferon gamma. Journal of Experimental Medicine, 178(1), 151–161.
Yang Chang, C., et al., Virus-stimulated neutrophils in the tumor microenvironment enhance T cell-mediated anti-tumor immunity. 2016. 7(27).
T Takeshima et al 2016 Key role for neutrophils in radiation-induced antitumor immune responses: Potentiation with G-CSF. 113 40 11300 11305
Eruslanov, E. B., et al. (2014). Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. The Journal of Clinical Investigation, 124(12), 5466–5480.
G Rosa de la et al 2008 Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. 180 10 6868 6876
Territo, M. C., et al. (1989). Monocyte-chemotactic activity of defensins from human neutrophils. The Journal of Clinical Investigation, 84(6), 2017–2020.
Yang, D., et al. (2000). Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. Journal of Leukocyte Biology, 68(1), 9–14.
Ethuin, F., et al. (2004). Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Laboratory Investigation, 84(10), 1363–1371.
Makarenkova, V. P., et al. (2006). CD11b<sup>+</sup>/Gr-1<sup>+</sup> Myeloid suppressor cells cause T cell dysfunction after traumatic stress. The Journal of Immunology, 176(4), 2085.
Bank, U., et al. (1999). Selective proteolytic cleavage of IL-2 receptor and IL-6 receptor ligand binding chains by neutrophil-derived serine proteases at foci of inflammation. Journal of Interferon & Cytokine Research, 19(11), 1277–1287.
K Tillack et al 2012 T Lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. 188 7 3150 3159
Ackermann, M. F., et al. (1989). Antitumor activity of murine neutrophils demonstrated by cytometric analysis. Cancer Research, 49(3), 528–532.
Blaisdell, A., et al. (2015). Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells. Cancer Cell, 28(6), 785–799.
Fields, G. B. (2019). Mechanisms of action of novel drugs targeting angiogenesis-promoting matrix metalloproteinases. Frontiers in Immunology, 10, 1278.
Finisguerra, V., et al. (2015). MET is required for the recruitment of anti-tumoural neutrophils. Nature, 522(7556), 349–353.
Matlung, H. L., et al. (2018). Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Reports, 23(13), 3946-3959.e6.
Güngör, N., et al. (2010). Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis, 25(2), 149–154.
Giese, M. A., Hind, L. E., & Huttenlocher, A. (2019). Neutrophil plasticity in the tumor microenvironment. Blood, 133(20), 2159–2167.
Deryugina, E. I., & Quigley, J. P. (2006). Matrix metalloproteinases and tumor metastasis. Cancer and Metastasis Reviews, 25(1), 9–34.
Bergers, G., et al. (2000). Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature Cell Biology, 2(10), 737–744.
Das, A., et al. (2017). MMP proteolytic activity regulates cancer invasiveness by modulating integrins. Scientific Reports, 7(1), 14219.
Houghton, A. M., et al. (2010). Neutrophil elastase–mediated degradation of IRS-1 accelerates lung tumor growth. Nature Medicine, 16(2), 219–223.
Hwang, W.-L., et al. (2019). Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. Journal of Hematology & Oncology, 12(1), 10.
Devalaraja, S., et al. (2020). Tumor-derived retinoic acid regulates intratumoral monocyte differentiation to promote immune suppression. Cell, 180(6), 1098-1114.e16.
Liu, B., Yan, L., & Zhou, M. (2019). Target selection of CAR T cell therapy in accordance with the TME for solid tumors. American journal of cancer research, 9(2), 228–241.
Ning, Y., et al., HDAC9 deficiency promotes tumor progression by decreasing the CD8(+) dendritic cell infiltration of the tumor microenvironment. J Immunother Cancer, 2020. 8(1).
Barry, K. C., et al. (2018). A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nature Medicine, 24(8), 1178–1191.
Zhou, S.-L., et al. (2016). Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to Sorafenib. Gastroenterology, 150(7), 1646-1658.e17.
MB Chen et al 2018 Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. 115 27 7022 7027
Kerfoot, S. M., et al. (2001). Exclusive neutrophil recruitment with oncostatin M in a human system. The American Journal of Pathology, 159(4), 1531–1539.
MM Queen et al 2005 Breast cancer cells stimulate neutrophils to produce oncostatin m: Potential implications for tumor progression. 65 19 8896 8904
Yang, Q., Yan, C., & Gong, Z. (2018). Interaction of hepatic stellate cells with neutrophils and macrophages in the liver following oncogenic kras activation in transgenic zebrafish. Scientific Reports, 8(1), 8495.
Zhou, Z., et al., Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. Journal for immunotherapy of cancer, 2021. 9(3): p. e001946.
Elinav, E., et al. (2013). Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nature Reviews Cancer, 13(11), 759–771.
Shang, K., et al., Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS One, 2012. 7(12): p. e51848.
Jamieson, T., et al. (2012). Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. The Journal of Clinical Investigation, 122(9), 3127–3144.
N Antonio et al 2015 The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. 34 17 2219 2236
Haqqani, A. S., Sandhu, J. K., & Birnboim, H. C. (2000). Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia, 2(6), 561–568.
Sandhu, J. K., et al. (2000). Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. The American Journal of Pathology, 156(2), 509–518.
Campregher, C., Luciani, M. G., & Gasche, C. (2008). Activated neutrophils induce an hMSH2-dependent G2/M checkpoint arrest and replication errors at a (CA)13-repeat in colon epithelial cells. Gut, 57(6), 780–787.
Wilson, C. L., et al. (2015). NFκB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nature Communications, 6, 6818.
Yan, C., et al. (2015). Stimulation of hepatocarcinogenesis by neutrophils upon induction of oncogenic kras expression in transgenic zebrafish. Journal of Hepatology, 63(2), 420–428.
Butin-Israeli, V., et al. (2019). Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. The Journal of Clinical Investigation, 129(2), 712–726.
Chang, S. H., et al. (2014). T helper 17 cells play a critical pathogenic role in lung cancer. Proceedings of the National Academic Sciences U S A, 111(15), 5664–5669.
Gong, L., et al. (2013). Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Molecular Cancer, 12(1), 154.
Takahashi, H., et al. (2010). Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell, 17(1), 89–97.
Deryugina, E. I., et al. (2014). Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia, 16(10), 771–788.
Jablonska, J., et al. (2010). Neutrophils responsive to endogenous IFN-β regulate tumor angiogenesis and growth in a mouse tumor model. The Journal of Clinical Investigation, 120(4), 1151–1164.
Coffelt, S. B., et al. (2015). IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature, 522(7556), 345–348.
McGary, C. T., Miele, M. E., & Welch, D. R. (1995). Highly metastatic 13762NF rat mammary adenocarcinoma cell clones stimulate bone marrow by secretion of granulocyte-macrophage colony-stimulating factor/interleukin-3 activity. The American journal of pathology, 147(6), 1668–1681.
MG Lechner DJ Liebertz AL Epstein 2010 Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. 185 4 2273 2284
Gabrilovich, D. I., & Nagaraj, S. (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology, 9(3), 162–174.
J-I Youn et al 2008 Subsets of myeloid-derived suppressor cells in tumor-bearing mice. 181 8 5791 5802
Powell, D., et al. (2018). Cxcr1 mediates recruitment of neutrophils and supports proliferation of tumor-initiating astrocytes in vivo. Science and Reports, 8(1), 13285.
Acharyya, S., et al. (2012). A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell, 150(1), 165–178.
Rodriguez, P. C., Quiceno, D. G., & Ochoa, A. C. (2006). l-arginine availability regulates T-lymphocyte cell-cycle progression. Blood, 109(4), 1568–1573.
Rodriguez, P. C., et al. (2002). Regulation of T cell receptor CD3ζ chain expression by<span class="small">l</span>-Arginine *. Journal of Biological Chemistry, 277(24), 21123–21129.
Feldmeyer, N., et al. (2012). Arginine deficiency leads to impaired cofilin dephosphorylation in activated human T lymphocytes. International Immunology, 24(5), 303–313.
Malmberg, K.-J., et al., Inhibition of activated/memory (CD45RO<sup>+</sup>) T cells by oxidative stress associated with block of NF-κB activation. 2001. 167(5): p. 2595–2601.
KA Gelderman et al 2006 T cell surface redox levels determine T cell reactivity and arthritis susceptibility. 103 34 12831 12836
Klemke, M., et al. (2008). Oxidation of cofilin mediates T cell hyporesponsiveness under oxidative stress conditions. Immunity, 29(3), 404–413.
Mantovani, A. (2009). The Yin-Yang of tumor-associated neutrophils. Cancer Cell, 16(3), 173–174.
Scapini, P., Bazzoni, F., & Cassatella, M. A. (2008). Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunology Letters, 116(1), 1–6.
Hahne, M., et al. (1998). APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. The Journal of experimental medicine, 188(6), 1185–1190.
Jabłońska, E., et al. (2012). A proliferation-inducing ligand (APRIL) in neutrophils of patients with oral cavity squamous cell carcinoma. European Cytokine Network, 23(3), 93–100.
Gruss, H. J., et al. (1994). Pleiotropic effects of the CD30 ligand on CD30-expressing cells and lymphoma cell lines. Blood, 83(8), 2045–2056.
Di Mitri, D., et al. (2014). Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature, 515(7525), 134–137.
Wang, G., et al. (2016). Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discovery, 6(1), 80–95.
Ma, X., et al. (2015). Definition of prostaglandin E2-EP2 signals in the colon tumor microenvironment that amplify inflammation and tumor growth. Cancer Research, 75(14), 2822–2832.
Liang, J., et al. (2014). Neutrophils promote the malignant glioma phenotype through S100A4. Clinical Cancer Research, 20(1), 187–198.
Song, W., et al. (2015). Infiltrating neutrophils promote renal cell carcinoma (RCC) proliferation via modulating androgen receptor (AR) → c-Myc signals. Cancer Letters, 368(1), 71–78.
Grégoire, M., et al. (2015). Neutrophils trigger a NF-κB dependent polarization of tumor-supportive stromal cells in germinal center B-cell lymphomas. Oncotarget, 6(18), 16471–16487.
Ramachandran, I. R., et al. (2016). Bone marrow PMN-MDSCs and neutrophils are functionally similar in protection of multiple myeloma from chemotherapy. Cancer Letters, 371(1), 117–124.
Hsu, B. E., et al. (2019). Immature low-density neutrophils exhibit metabolic flexibility that facilitates breast cancer liver metastasis. Cell Reports, 27(13), 3902-3915.e6.
Guglietta, S., et al. (2016). Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nature Communications, 7(1), 11037.
Hsu, B. E., et al. (2020). C3a elicits unique migratory responses in immature low-density neutrophils. Oncogene, 39(12), 2612–2623.
Gomes, T., et al., IL-1β blockade attenuates thrombosis in a neutrophil extracellular trap-dependent breast cancer model. 2019. 10(2088).
HO Yazdani et al 2019 Neutrophil extracellular traps drive mitochondrial homeostasis in tumors to augment growth. 79 21 5626 5639
Zhu, B., et al., NF-κB and neutrophil extracellular traps cooperate to promote breast cancer progression and metastasis. Experimental Cell Research, 2021. 405(2): p. 112707.
Zhang, X., et al. (2016). Neutrophils in cancer development and progression: Roles, mechanisms, and implications (Review). International Journal of Oncology, 49(3), 857–867.
Zhang, J., et al. (2016). Circulating tumor-associated neutrophils (cTAN) contribute to circulating tumor cell survival by suppressing peripheral leukocyte activation. Tumor Biology, 37(4), 5397–5404.
Koh, Y. W., et al. (2016). Baseline neutrophil-lymphocyte ratio is associated with baseline and subsequent presence of brain metastases in advanced non-small-cell lung cancer. Science and Reports, 6, 38585.
Wellenstein, M. D., et al. (2019). Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature, 572(7770), 538–542.
Singh, S., et al. (2020). Loss of ELF5-FBXW7 stabilizes IFNGR1 to promote the growth and metastasis of triple-negative breast cancer through interferon-γ signalling. Nature Cell Biology, 22(5), 591–602.
Markman, J. L., et al. (2020). Loss of testosterone impairs anti-tumor neutrophil function. Nature Communications, 11(1), 1613.
Quail, D. F., et al. (2017). Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nature Cell Biology, 19(8), 974–987.
Charan, M., et al. (2020). Tumor secreted ANGPTL2 facilitates recruitment of neutrophils to the lung to promote lung pre-metastatic niche formation and targeting ANGPTL2 signaling affects metastatic disease. Oncotarget, 11(5), 510–522.
Jackstadt, R., et al. (2019). Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell, 36(3), 319-336.e7.
Janiszewska, M., et al. (2019). Subclonal cooperation drives metastasis by modulating local and systemic immune microenvironments. Nature Cell Biology, 21(7), 879–888.
Liang, W., Li, Q., & Ferrara, N. (2018). Metastatic growth instructed by neutrophil-derived transferrin. Proceedings of the National Academic Sciences U S A, 115(43), 11060–11065.
Freisinger, C.M. and A. Huttenlocher, Live imaging and gene expression analysis in zebrafish identifies a link between neutrophils and epithelial to mesenchymal transition. PLoS One, 2014. 9(11): p. e112183.
Hu, P., et al. (2015). Intratumoral neutrophil granulocytes contribute to epithelial-mesenchymal transition in lung adenocarcinoma cells. Tumour Biology, 36(10), 7789–7796.
Wang, Y., et al. (2019). Tumor-contacted neutrophils promote metastasis by a CD90-TIMP-1 Juxtacrine-paracrine loop. Clinical Cancer Research, 25(6), 1957–1969.
Lin, C., et al. (2015). Infiltrating neutrophils increase bladder cancer cell invasion via modulation of androgen receptor (AR)/MMP13 signals. Oncotarget, 6(40), 43081–43089.
Park, J., et al., Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Science Translational Medicine, 2016. 8(361): p. 361ra138.
Abdol Razak, N., O. Elaskalani, and P. Metharom, Pancreatic cancer-induced neutrophil extracellular traps: a potential contributor to cancer-associated thrombosis. International Journal of Molecular Science, 2017. 18(3).
Lee, W., et al. (2019). Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. Journal of Experimental Medicine, 216(1), 176–194.
Takesue, S., et al. (2020). Neutrophil extracellular traps promote liver micrometastasis in pancreatic ductal adenocarcinoma via the activation of cancer-associated fibroblasts. International Journal of Oncology, 56(2), 596–605.
Tecchio, C., et al. (2013). On the cytokines produced by human neutrophils in tumors. Seminars in Cancer Biology, 23(3), 159–170.
Queen, M. M., et al. (2005). Breast cancer cells stimulate neutrophils to produce oncostatin M: Potential implications for tumor progression. Cancer Research, 65(19), 8896–8904.
Junk, D. J., et al. (2017). Oncostatin M promotes cancer cell plasticity through cooperative STAT3-SMAD3 signaling. Oncogene, 36(28), 4001–4013.
Wislez, M., et al. (2003). Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: Role in tumor progression and death. Cancer Research, 63(6), 1405–1412.
Imai, Y., et al. (2005). Neutrophils enhance invasion activity of human cholangiocellular carcinoma and hepatocellular carcinoma cells: An in vitro study. Journal of Gastroenterology and Hepatology, 20(2), 287–293.
Ikushima, H., & Miyazono, K. (2010). Cellular context-dependent “colors” of transforming growth factor-beta signaling. Cancer Science, 101(2), 306–312.
Yang, L., Pang, Y., & Moses, H. L. (2010). TGF-beta and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends in Immunology, 31(6), 220–227.
Aoyagi, Y., et al. (2004). Overexpression of TGF-beta by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer. British Journal of Cancer, 91(7), 1316–1326.
SenGupta, S., et al., Triple-negative breast cancer cells recruit neutrophils by secreting TGF-β and CXCR2 ligands. 2021. 12(973).
Waugh, D. J., & Wilson, C. (2008). The interleukin-8 pathway in cancer. Clinical Cancer Research, 14(21), 6735–6741.
Palena, C., Hamilton, D. H., & Fernando, R. I. (2012). Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncology, 8(6), 713–722.
Konrad, F. M., et al. (2019). How adhesion molecule patterns change while neutrophils traffic through the lung during inflammation. Mediators of Inflammation, 2019, 1208086.
Gil, C. D., et al. (2006). Interaction of human neutrophils with endothelial cells regulates the expression of endogenous proteins annexin 1, galectin-1 and galectin-3. Cell Biology International, 30(4), 338–344.
Strell, C., et al. (2010). Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Experimental Cell Research, 316(1), 138–148.
Spicer, J. D., et al. (2012). Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Research, 72(16), 3919–3927.
Szczerba, B. M., et al. (2019). Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature, 566(7745), 553–557.
Spiegel, A., et al. (2016). Neutrophils suppress intraluminal nk cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discovery, 6(6), 630–649.
Nourshargh, S., et al. (1995). Interleukin-1-induced leukocyte extravasation across rat mesenteric microvessels is mediated by platelet-activating factor. Blood, 85(9), 2553–2558.
Rayes, R. F., et al. (2020). Neutrophil Extracellular trap-associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. The Journal of Immunology, 204(8), 2285–2294.
S Xiong et al 2021 Neutrophils in cancer carcinogenesis and metastasis. 14 1 1 17
Rayes, R.F., et al., Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight, 2019. 5(16).
Yang, L. Y., et al. (2020). Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. Journal of Hematology & Oncology, 13(1), 3.
Rocks, N., et al. (2019). Ozone-primed neutrophils promote early steps of tumour cell metastasis to lungs by enhancing their NET production. Thorax, 74(8), 768–779.
Inoue, M., et al. (2018). Plasma redox imbalance caused by albumin oxidation promotes lung-predominant NETosis and pulmonary cancer metastasis. Nature Communications, 9(1), 5116.
Nozawa, H., Chiu, C., & Hanahan, D. (2006). Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of National Academic Sciences U S A, 103(33), 12493–12498.
Bekes, E. M., et al. (2011). Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. American Journal of Pathology, 179(3), 1455–1470.
Keane, M. P., et al. (2004). Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. The Journal of Immunology, 172(5), 2853–2860.
Yang, L., et al. (2004). Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell, 6(4), 409–421.
Coussens, L. M., et al. (2000). MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell, 103(3), 481–490.
Shojaei, F., et al. (2008). Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proceedings of National Academic Sciences U S A, 105(7), 2640–2645.
Ferrara, N., Gerber, H. P., & LeCouter, J. (2003). The biology of VEGF and its receptors. Nature Medicine, 9(6), 669–676.
Jablonska, J., et al. (2010). Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. The Journal of Clinical Investigation, 120(4), 1151–1164.
Kusumanto, Y. H., et al. (2003). Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis, 6(4), 283–287.
Jablonska, E., et al. (2002). VEGF in the culture of PMN and the serum in oral cavity cancer patients. Oral Oncology, 38(6), 605–609.
Zhong, C., et al. (2009). Characterization and regulation of bv8 in human blood cells. Clinical Cancer Research, 15(8), 2675–2684.
Kuang, D.-M., et al. (2011). Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. Journal of Hepatology, 54(5), 948–955.
Ardi, V.C., et al., Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. 2007. 104(51): p. 20262–20267.
Egeblad, M., & Werb, Z. (2002). New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer, 2(3), 161–174.
Gordon-Weeks, A. N., et al. (2017). Neutrophils promote hepatic metastasis growth through fibroblast growth factor 2-dependent angiogenesis in mice. Hepatology, 65(6), 1920–1935.
Belikov, A. V., Schraven, B., & Simeoni, L. (2015). T cells and reactive oxygen species. Journal of Biomedical Science, 22(1), 85.
Galli, S. J., Borregaard, N., & Wynn, T. A. (2011). Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nature Immunology, 12(11), 1035–1044.
Rotondo, R., et al. (2009). IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. International Journal of Cancer, 125(4), 887–893.
Yachimovich-Cohen, N., et al. (2010). Human embryonic stem cells suppress T cell responses via arginase I-dependent mechanism. The Journal of Immunology, 184(3), 1300–1308.
Waldron, T.J., et al., Myeloid derived suppressor cells: targets for therapy. Oncoimmunology, 2013. 2(4): p. e24117.
Yang, T. H., et al. (2018). Membrane-associated proteinase 3 on granulocytes and acute myeloid leukemia inhibits T cell proliferation. The Journal of Immunology, 201(5), 1389–1399.
Schmielau, J., & Finn, O. J. (2001). Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Research, 61(12), 4756–4760.
Sippel, T. R., et al. (2011). Neutrophil degranulation and immunosuppression in patients with GBM: Restoration of cellular immune function by targeting arginase I. Clinical Cancer Research, 17(22), 6992–7002.
Wang, T.-t., et al., Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. 2017. 66(11): p. 1900–1911.
He, G., et al. (2015). Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. Journal of Experimental & Clinical Cancer Research, 34(1), 141.
el-Hag, A. and R.A. Clark, Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. The Journal of Immunology, 1987. 139(7): p. 2406–13.
Yang, J., et al. (2018). Loss of CXCR4 in myeloid cells enhances antitumor immunity and reduces melanoma growth through NK cell and FASL mechanisms. Cancer Immunology Research, 6(10), 1186–1198.
Michaeli, J., et al., Tumor-associated neutrophils induce apoptosis of non-activated CD8 T-cells in a TNFα and NO-dependent mechanism, promoting a tumor-supportive environment. Oncoimmunology, 2017. 6(11): p. e1356965.
Zhou, M., et al., Tumor-released autophagosomes induce IL-10-producing B cells with suppressive activity on T lymphocytes via TLR2-MyD88-NF-κB signal pathway. Oncoimmunology, 2016. 5(7): p. e1180485.
Gao, R., et al., Tumor cell-released autophagosomes (TRAP) enhance apoptosis and immunosuppressive functions of neutrophils. Oncoimmunology, 2018. 7(6): p. e1438108.
Cedrés, S., et al. (2012). Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clinical and Translational Oncology, 14(11), 864–869.
Yang, S. Z., et al. (2015). Elevated levels of preoperative circulating CD44+ lymphocytes and neutrophils predict poor survival for non-small cell lung cancer patients. Clinica Chimica Acta, 439, 172–177.
Zhang, H., et al., Prognostic significance of combination of preoperative platelet count and neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small cell lung cancer: based on a large cohort study. PLoS One, 2015. 10(5): p. e0126496.
Orditura, M., et al., Neutrophil to lymphocyte ratio (NLR) for prediction of distant metastasis-free survival (DMFS) in early breast cancer: a propensity score-matched analysis. ESMO Open, 2016. 1(2): p. e000038.
HK Jensen et al 2009 Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. 27 28 4709 4717
Trellakis, S., et al. (2011). Polymorphonuclear granulocytes in human head and neck cancer: Enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. International Journal of Cancer, 129(9), 2183–2193.
Asaoka, T., et al. (2016). Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology, 16(3), 434–440.
Rao, H.L., et al., Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients' adverse prognosis. PLoS One, 2012. 7(1): p. e30806.
Romano, A., et al. (2018). Prognostic meaning of neutrophil to lymphocyte ratio (NLR) and lymphocyte to monocyte ration (LMR) in newly diagnosed Hodgkin lymphoma patients treated upfront with a PET-2 based strategy. Annals of Hematology, 97(6), 1009–1018.
Ferrucci, P. F., et al. (2015). Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. British Journal of Cancer, 112(12), 1904–1910.
Galdiero, M. R., et al. (2016). Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. International Journal of Cancer, 139(2), 446–456.
Li, Y., et al. (2017). Preoperative NLR for predicting survival rate after radical resection combined with adjuvant immunotherapy with CIK and postoperative chemotherapy in gastric cancer. Journal of Cancer Research and Clinical Oncology, 143(5), 861–871.
Nakaya, A., et al. (2018). Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. International Journal of Clinical Oncology, 23(4), 634–640.
Zhou, S. L., et al. (2012). Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology, 56(6), 2242–2254.
Jensen, T. O., et al. (2012). Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer, 118(9), 2476–2485.
AD Gregory 2011 and A McGarry Houghton, Tumor-associated neutrophils: New targets for cancer therapy. 71 7 2411 2416
Ruffini, P.A., The CXCL8-CXCR1/2 axis as a therapeutic target in breast cancer stem-like cells. 2019. 9(40).
Lemos, H. P., et al. (2009). Prostaglandin mediates IL-23/IL-17-induced neutrophil migration in inflammation by inhibiting IL-12 and IFNgamma production. Proceedings of National Academic Sciences U S A, 106(14), 5954–5959.
Highfill, S.L., et al., Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Science Translational Medicine, 2014. 6(237): p. 237ra67.
Gebhardt, C., et al. (2015). Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clinical Cancer Research, 21(24), 5453–5459.
Nam, J. S., et al. (2008). An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Research, 68(10), 3835–3843.
Bhola, N. E., et al. (2013). TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. The Journal of Clinical Investigation, 123(3), 1348–1358.
Pang, Y., et al. (2013). TGF-β signaling in myeloid cells is required for tumor metastasis. Cancer Discovery, 3(8), 936–951.
Sun, R., et al. (2014). Neutrophils with protumor potential could efficiently suppress tumor growth after cytokine priming and in presence of normal NK cells. Oncotarget, 5(24), 12621–12634.
Andzinski, L., et al. (2016). Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. International Journal of Cancer, 138(8), 1982–1993.
Shrestha, S., et al., Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonist attenuate tumor growth via polarization of neutrophils toward an antitumor phenotype. Oncoimmunology, 2016. 5(1): p. e1067744.
Teijeira, Á., et al. (2020). CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity, 52(5), 856-871.e8.
Sandhu, J.K., et al., Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. 2000. 156(2): p. 509–518.
V Butin-Israeli et al 2019 Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. 129 2 712 726
Guglietta, S., et al., Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. 2016. 7(1): p. 1–14.
Awaji, M., et al., CXCR2 signaling promotes secretory cancer‐associated fibroblasts in pancreatic ductal adenocarcinoma. 2020. 34(7): p. 9405–9418.
Zhang, X., et al., Inflammation‐induced S100A8 activates I d3 and promotes colorectal tumorigenesis. 2015. 137(12): p. 2803–2814.
C Yan et al 2015 Stimulation of hepatocarcinogenesis by neutrophils upon induction of oncogenic kras expression in transgenic zebrafish. 63 2 420 428
Houghton, A.M., et al., Neutrophil elastase–mediated degradation of IRS-1 accelerates lung tumor growth. 2010. 16(2): p. 219–223.
Liang, J., et al., Neutrophils promote the malignant glioma phenotype through S100A4. 2014. 20(1): p. 187–198.
Cheng, S.P., et al., Overexpression of chitinase‐3‐like protein 1 is associated with structural recurrence in patients with differentiated thyroid cancer. 2020. 252(2): p. 114–124.
Yang, R., et al., Neutrophil elastase enhances the proliferation and decreases apoptosis of leukemia cells via activation of PI3K/Akt signaling. 2016. 13(5): p. 4175–4182.
Powell, D., et al., Cxcr1 mediates recruitment of neutrophils and supports proliferation of tumor-initiating astrocytes in vivo. 2018. 8(1): p. 1–12.
J Cools-Lartigue et al 2013 Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. 123 8 3446 3458
Yang, L., et al., DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. 2020. 583(7814): p. 133–138.
L-Y Yang et al 2020 Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. 13 1 1 15
Kwon, C.H., et al., S100A8 and S100A9 promotes invasion and migration through p38 mitogen-activated protein kinase-dependent NF-κB activation in gastric cancer cells. 2013. 35(3): p. 226–234.
Liu, Y., et al., Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. 2016. 30(2): p. 243–256.
Wang, Z., et al., Tumor-derived HMGB1 induces CD62Ldim neutrophil polarization and promotes lung metastasis in triple-negative breast cancer. 2020. 9(9): p. 1–17.
Kolonin, M.G., et al., Interaction between tumor cell surface receptor RAGE and proteinase 3 mediates prostate cancer metastasis to bone. 2017. 77(12): p. 3144–3150.
Deryugina, E., et al., Neutrophil elastase facilitates tumor cell intravasation and early metastatic events. 2020. 23(12): p. 101799.
Wilson, T.J., et al., Cathepsin G-mediated enhanced TGF-β signaling promotes angiogenesis via upregulation of VEGF and MCP-1. 2010. 288(2): p. 162–169.
E Pieterse et al 2017 Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. 37 7 1371 1379
Deryugina, E.I., et al., Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. 2014. 16(10): p. 771–788.
N Feldmeyer et al 2012 Arginine deficiency leads to impaired cofilindephosphorylation in activated human T lymphocytes. 24 5 303 313
Malmberg, K.-J., et al., Inhibition of activated/memory (CD45RO+) T cells by oxidative stress associated with block of NF-κB activation. 2001. 167(5): p. 2595–2601.
Yang, T.-H., et al., Membrane-associated proteinase 3 on granulocytes and acute myeloid leukemia inhibits T cell proliferation. 2018. 201(5): p. 1389–1399.
Spiegel, A., et al., Neutrophils suppress intraluminal NK cell–mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. 2016. 6(6): p. 630–649.
He, G., et al., Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. 2015. 34(1): p. 1–11.
Michaeli, J., et al., Tumor-associated neutrophils induce apoptosis of non-activated CD8 T-cells in a TNFα and NO-dependent mechanism, promoting a tumor-supportive environment. 2017. 6(11): p. e1356965.
Shang, A., et al., Exosomal KRAS mutation promotes the formation of tumor-associated neutrophil extracellular traps and causes deterioration of colorectal cancer by inducing IL-8 expression. 2020. 18(1): p. 1–14.
J Cedervall et al 2015 Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. 75 13 2653 2662
Bang, O.Y., et al., Circulating DNAs, a marker of neutrophil extracellular traposis and cancer-related stroke: the OASIS-Cancer Study. 2019. 50(10): p. 2944–2947.
Wolach, O., et al., Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. 2018. 10(436): p. eaan8292.
Perego, M., et al., Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. 2020. 12(572): p. eabb5817.
Albrengues, J., et al., Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. 2018. 361(6409): p. eaao4227.
S-Y Park J-SJE Nam M Medicine 2020 The force awakens: Metastatic dormant cancer cells. 52 4 569 581
Zhou, S.-L., et al., Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. 2016. 150(7): p. 1646–1658. e17.
J Incio et al 2016 Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. 6 8 852 869
Zhang, Y., et al., Interleukin-17–induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. 2020. 217(12).
Bui, T.M., L.K. Yalom, and R.J.E.o.o.t.t. Sumagin, Tumor-associated neutrophils: orchestrating cancer pathobiology and therapeutic resistance. 2021. 25(7): p. 573–583.
Wang, Y., et al. (2014). Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal immunology, 7(5), 1106–1115.
Bellocq, A., et al. (1998). Neutrophil alveolitis in bronchioloalveolar carcinoma: Induction by tumor-derived interleukin-8 and relation to clinical outcome. The American journal of pathology, 152(1), 83–92.
Ibrahim, M.L., et al., Myeloid-derived suppressor cells produce IL-10 to elicit DNMT3b-dependent IRF8 silencing to promote colitis-associated colon tumorigenesis. Cell reports, 2018. 25(11): p. 3036–3046. e6.
Li, T.-J., et al. (2017). Interleukin-17–producing neutrophils link inflammatory stimuli to disease progression by promoting angiogenesis in gastric cancer. Clinical Cancer Research, 23(6), 1575–1585.
Jablonska, E., et al. (2005). VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer. Cytokine, 30(3), 93–99.
Wada, Y., et al. (2007). Neutrophil elastase induces cell proliferation and migration by the release of TGF-α, PDGF and VEGF in esophageal cell lines. Oncology reports, 17(1), 161–167.
Zhang, X., & Xu, W. (2017). Neutrophils diminish T-cell immunity to foster gastric cancer progression: The role of GM-CSF/PD-L1/PD-1 signalling pathway. Gut, 66(11), 1878–1880.
Fridlender, Z. G., et al. (2009). Polarization of tumor-associated neutrophil phenotype by TGF-β:“N1” versus “N2” TAN. Cancer Cell, 16(3), 183–194.
Aoyagi, Y., et al. (2004). Overexpression of TGF-β by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer. British journal of cancer, 91(7), 1316–1326.
Dumitru, C. A., et al. (2012). A novel p38-MAPK signaling axis modulates neutrophil biology in head and neck cancer. Journal of leukocyte biology, 91(4), 591–598.
Tsuda, Y., et al., An immunosuppressive subtype of neutrophils identified in patients with hepatocellular carcinoma. Journal of clinical biochemistry and nutrition, 2012: p. 12–32.
Eruslanov, E., et al. (2012). Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. International journal of cancer, 130(5), 1109–1119.
Yan, H. H., et al. (2015). CCL9 induced by TGFβ signaling in myeloid cells enhances tumor cell survival in the premetastatic organ. Cancer research, 75(24), 5283–5298.
Mishalian, I., et al. (2014). Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17—A new mechanism of impaired antitumor immunity. International journal of cancer, 135(5), 1178–1186.
Sasaki, S., et al. (2018). Involvement of prokineticin 2–expressing neutrophil infiltration in 5-fluorouracil–induced aggravation of breast cancer metastasis to lung. Molecular cancer therapeutics, 17(7), 1515–1525.
Ibrahim, S. A., et al. (2015). Breast cancer associated a2 isoform vacuolar ATPase immunomodulates neutrophils: Potential role in tumor progression. Oncotarget, 6(32), 33033.
Dumitru, C. A., et al. (2011). Tumor-derived macrophage migration inhibitory factor modulates the biology of head and neck cancer cells via neutrophil activation. International Journal of Cancer, 129(4), 859–869.
Khan, S., et al. (2020). Role of neutrophils and myeloid-derived suppressor cells in glioma progression and treatment resistance. International journal of molecular sciences, 21(6), 1954.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahmud, Z., Rahman, A., Mishu, I.D. et al. Mechanistic insights into the interplays between neutrophils and other immune cells in cancer development and progression. Cancer Metastasis Rev 41, 405–432 (2022). https://doi.org/10.1007/s10555-022-10024-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-022-10024-8